Categories
Change Password!
Reset Password!


This post hoc analysis aimed to examine the sustained responses of 50% or more, 75% or more, or a complete decrease (100%) in monthly migraine days (MMDs) with the use of Atogepant (oral calcitonin gene-related peptide receptor antagonist) over 12 and 52 weeks.
A high proportion of episodic migraine patients who achieve a 50% or more reduction in migraine days per month within the first month of Atogepant treatment maintain this improvement throughout the treatment phase.
This post hoc analysis aimed to examine the sustained responses of 50% or more, 75% or more, or a complete decrease (100%) in monthly migraine days (MMDs) with the use of Atogepant (oral calcitonin gene-related peptide receptor antagonist) over 12 and 52 weeks.
The data from the ADVANCE, a 12-week randomized trial and open-label long-term safety (LTS) trial was examined and assessed. Atogepant (given in 10, 30, and 60 mg doses once daily) was compared with a placebo for preventing episodic migraines in the ADVANCE trial, and the LTS trial evaluated daily administration of Atogepant 60 mg over 52 weeks. For safety concerns, Atogepant as 60 mg dose was used.
An initial response was described as a decrease of ≥50%, ≥75%, or 100% from the starting MMDs during the first month for ADVANCE or the first quarter for the LTS trial. The percentage of participants who maintained a response exceeding each threshold through subsequent months (for ADVANCE) or quarters (for the LTS trial) was estimated.
As found, the sustained response rates during months 2 and 3 varied with dose in the ADVANCE trial (Table 1):
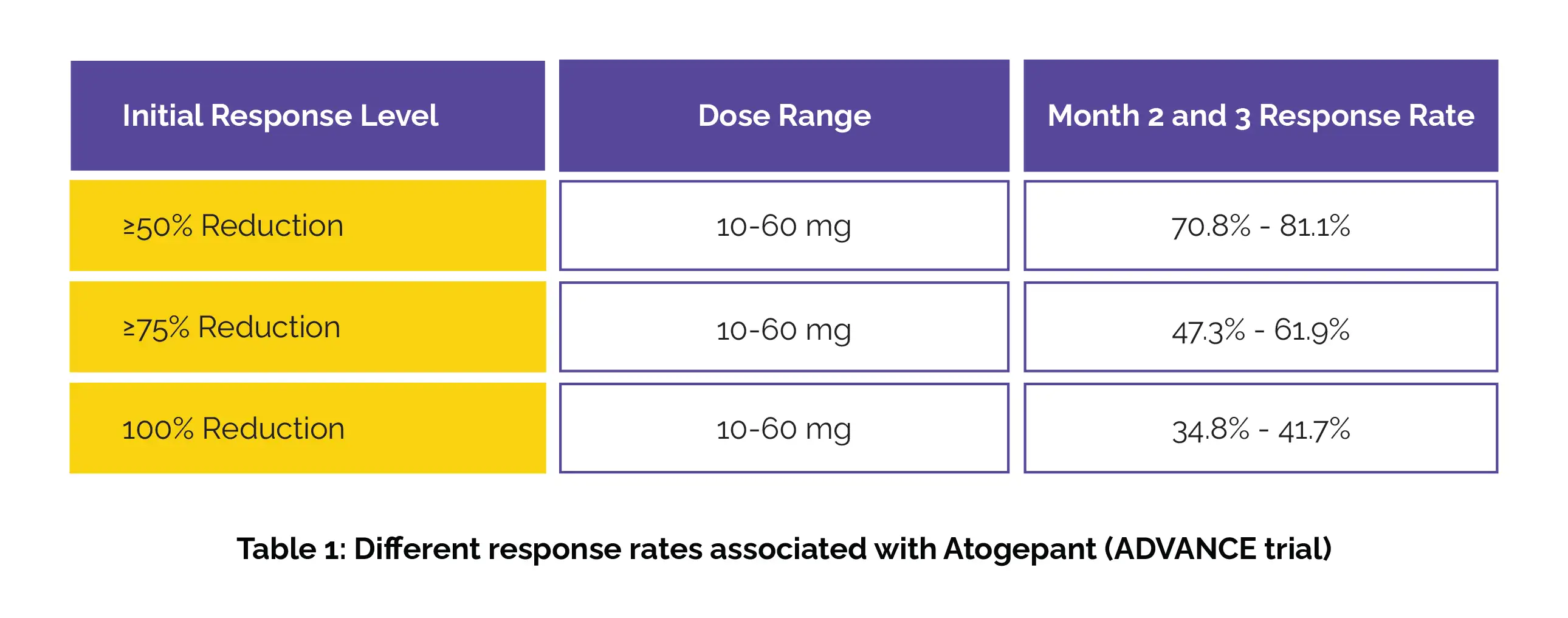
Among those with an initial ≥75% or 100% response in month 1, over 79% maintained at least a 50% response in months 2 and 3. The sustained response rates through quarters 2, 3, and 4 in the LTS trial have been shown in Table 2:
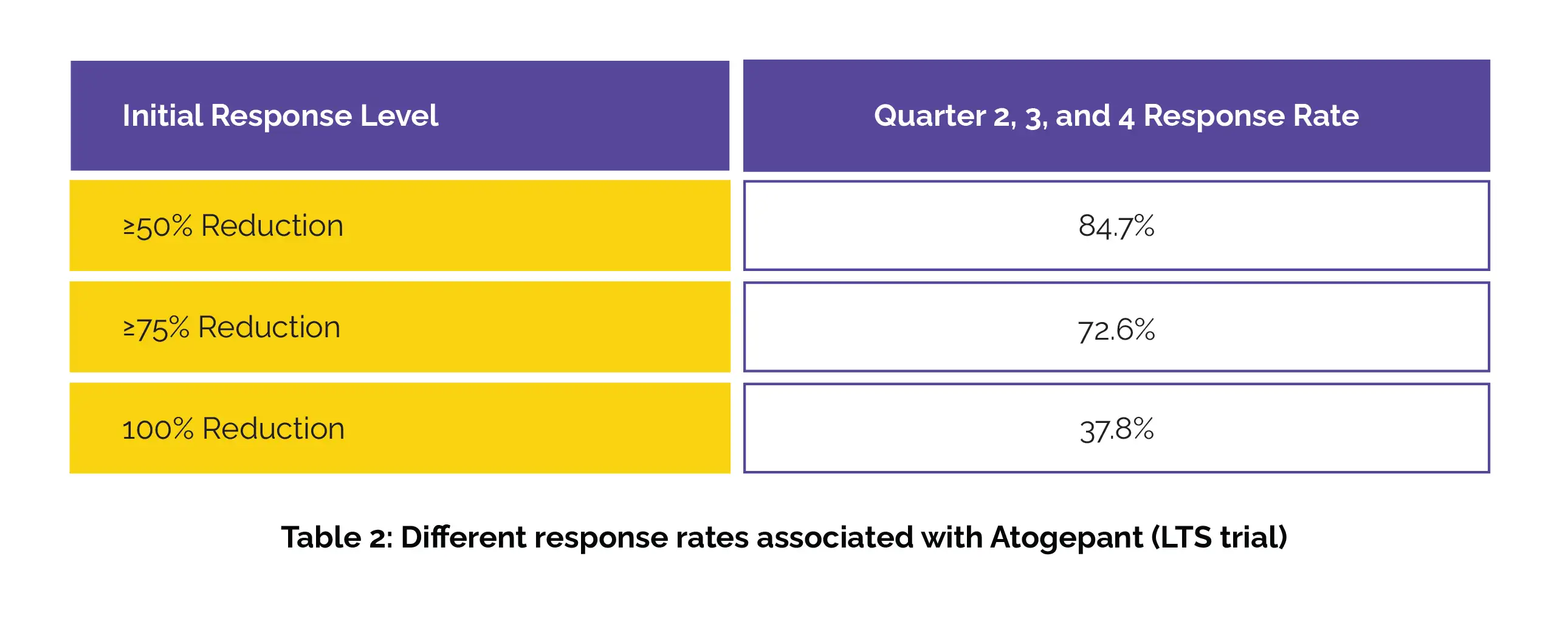
Among those who had an initial ≥75% or 100% response in quarter 1, more than 90% sustained at least a 50% response through quarters 2, 3, and 4.
More than 70% of participants who initially responded to Atogepant treatment continued to show long-term effectiveness with continued use.
The Journal of Headache and Pain
Sustained response to Atogepant in episodic migraine: post hoc analyses of a 12-week randomized trial and a 52-week long-term safety trial
Richard B. Lipton et al.
Comments (0)