Categories
Change Password!
Reset Password!


Globally, low back pain (LBP) is a predominant musculoskeletal ailment contributing significantly to disability and socioeconomic burden. The occurrence of initial episodes reaches 15%, and within one year, 80% of individuals encounter a recurrence mimicking their original activity.
NSAIDs, NSAIDs + paracetamol, and myorelaxants effectively provide symptomatic relief in acute low back pain while reducing disability in patients.
Globally, low back pain (LBP) is a predominant musculoskeletal ailment contributing significantly to disability and socioeconomic burden. The occurrence of initial episodes reaches 15%, and within one year, 80% of individuals encounter a recurrence mimicking their original activity. In the United States, 25% of surveyed patients, when asked about common sources of pain, mentioned experiencing LBP in the three months preceding the questionnaire.
Acute LBP is characterized by symptoms lasting 2–12 weeks, yet a significant portion, up to 60% of patients, progresses to chronic LBP. Since initial episodes commonly resolve on their own, many patients do not actively seek medical attention, potentially contributing to the elevated recurrence rate. Despite literature recognizing up to 16 distinct evolution patterns following acute LBP, specific risk factors facilitating the transition from acute to chronic LBP remain elusive. Nonetheless, psychological factors have been demonstrated to influence this progression.
The limited interaction between patients and medical care personnel could be a contributing factor to the scarcity of literature and research on acute LBP, in contrast to chronic LBP. Consequently, conclusions in this field often lack consensus. As per existing guidelines, the primary approach to treatment involves non-pharmacological methods, reserving pharmacological management for cases where patients express a preference or when initial non-pharmacological interventions prove inadequate. However, the evidence supporting non-pharmacological treatment for acute LBP is categorized as low to moderate.
These interventions encompass local heat, massage/manipulations, and acupuncture. Concerning pharmacological treatment, although there is insufficient evidence endorsing opioid usage for acute LBP, these medications are prescribed to nearly 14% of individuals reporting acute LBP. However, a substantial percentage, up to 95%, of people undergoing opioid treatment encounter a minimum of one adverse reaction. In the long term, the development of tolerance and opioid-elicited hyperalgesia is also a potential outcome. Recent research has also indicated potential negative effects over an extended period in individuals utilizing opioids for acute LBP. Pharmacological treatment has the potential to alleviate symptoms in cases of acute LBP.
RATIONALE BEHIND RESEARCH
There is a dearth of comprehensive understanding regarding the optimal management of acute LBP. The majority of meta-analyses and systematic reviews on this subject highlight an insufficiency of data, leading to inconclusive conclusions. Therefore, this study was conducted.
OBJECTIVE
This systematic review (level I of evidence) aimed to investigate nonopioid pharmacological management of acute LBP and identify the most effective drugs.
Literature search
Databases such as Web of Science, Scopus, and PubMed were retrieved utilizing a combination of specified keywords through Boolean operators AND/OR: (acute) AND (lumbar OR low) AND (back pain OR pain) AND (administration OR management OR treatment OR outcome) AND (myorelaxants OR muscle relaxants OR NSAIDs OR nonsteroidal anti-inflammatory drugs OR paracetamol OR acetaminophen) AND (visual analogue scale OR VAS OR numeric rating system OR NRS). The same search criteria were applied to Web of Science, Google Scholar, and Embase with no temporal restrictions. Notably, the search was restricted exclusively to randomized controlled trials (RCTs).
Inclusion criteria
This systematic review (following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses [PRISMA] statement) encompassed the following studies:
Exclusion criteria
Study Selection and Data Extraction
Two authors autonomously conducted the initial database exploration. All generated titles were reviewed, and when appropriate, the corresponding abstracts were examined. The full text of abstracts aligning with the topic was then evaluated. Subsequently, both authors entered the extracted data into Microsoft Office Excel. To ensure accuracy during data extraction, both tables were thoroughly double-checked, with the two authors cross-verifying all datasheets.
The bibliography of full-text articles was also scrutinized for eligibility. All incorporated studies were gathered into a folder and arranged in alphabetical order. Any duplicate entries were removed. In instances of disagreements concerning the literature search or bias assessment, a third author facilitated the discussion until a consensus was reached among all three authors. Demographic information, including the author's name, publication year, journal name, duration of symptoms prior to the treatment, and follow-up duration, was extracted.
The sample size, along with the mean age and the number of female patients, was also obtained. The focus of interest was to assess differences in disability and pain between the drugs. Pain was assessed using either the visual analogue scale (VAS) or the numeric rating system (NRS). Since VAS and NRS are interchangeable for a dependable evaluation of LBP, they were treated as synonyms for the purpose of this study. Utilizing the Roland Morris Questionnaire (RMQ), the measurement of disability was done.
Data and Statistical Analysis
Statistical analyses were executed by a single author utilizing the IBM Statistical Package for Social Sciences (SPSS) software version 25. Baseline comparability was assessed using the one-way analysis of variance (ANOVA) and mean difference (MD). The MD effect measure was employed to estimate the alteration between the baseline and the last follow-up for continuous data. In all comparisons, the standard error and T value were assessed. A confidence interval of 0.95 was applied to all comparisons. The paired t-test was employed, and values of p < 0.05 were deemed statistically significant.
Risk of Bias and Quality Assessment
The evaluation of the risk of bias utilized Review Manager 5.3 software from the Nordic Cochrane Collaboration in Copenhagen. Two reviewers appraised the bias risk in the identified studies. Various outcomes, including reporting, attrition, performance, detection, selection, and other biases were assessed.
Study outcomes
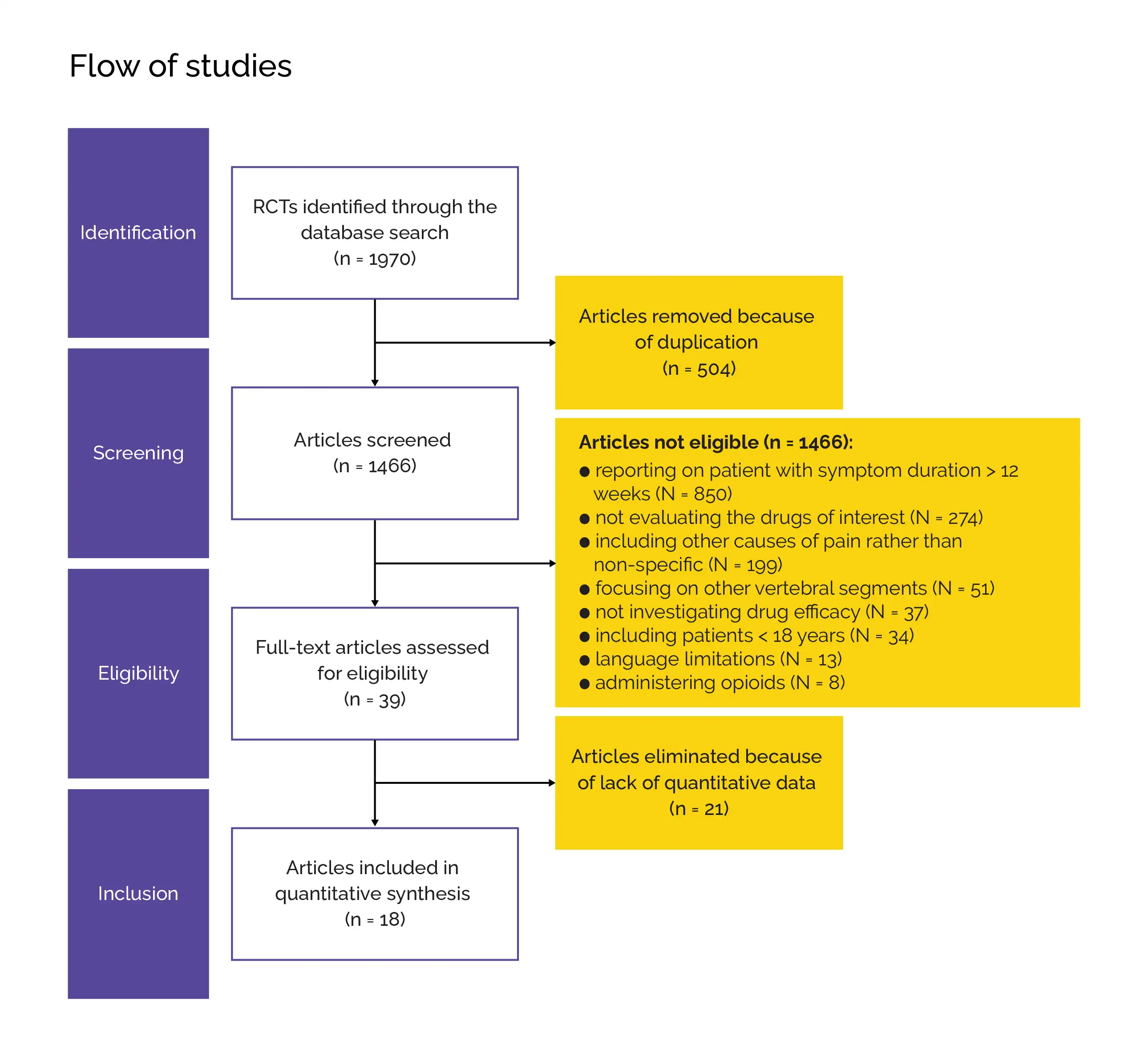
Outcomes
Study and participant characteristics:
Study quality:
Effect of intervention on the outcome:
This systematic review exclusively addressed the management of non-specific acute LBP. According to key findings, myorelaxants and NSAIDs proved to be valuable in alleviating pain and disability in cases of acute LBP after around a week. Combining NSAIDs with paracetamol showed more remarkable enhancement compared to using NSAIDs alone, although the sole use of paracetamol did not result in any noteworthy betterment in clinical outcomes. Notably, the administration of a placebo did not demonstrate effectiveness in pain reduction.
When managing patients battling acute LBP, it was essential to exclude potential specific causes of pain that might necessitate specific diagnostics or interventions, such as a history of recent trauma or cancer. The identification of potential red flags should consistently form a cornerstone in acute LBP evaluation.
In light of the spontaneous alleviation of symptoms in the majority of patients dealing with acute LBP, the actual effectiveness of pharmacological interventions remained uncertain. It was noteworthy that the placebo group did not show a substantial change from baseline to the final follow-up, supporting the idea that pharmacological management contributed to symptom improvement. Additionally, for those individuals who did not experience sufficient benefits from non-pharmacological approaches, there was a need to provide a therapeutic alternative.
While this study exclusively addressed the pharmacological treatment of acute LBP, it was crucial to underscore that resorting to medications should always be a secondary approach, employed after other non-pharmacological and noninvasive therapies had been deemed insufficient. The results presented aligned with the recommendations of Quaseem et al in which NSAIDs effectively played a role in combatting acute LBP, whereas the use of paracetamol alone did not yield better results than placebo.
However, the guidelines did not contemplate the combination of paracetamol and NSAIDs or the utilization of myorelaxants—two therapeutic modalities that this current study identified as effective. Concerning NSAIDs, another review affirmed the effectiveness of these medications in addressing acute LBP. Although the present analysis couldn't distinguish between various classes of NSAIDs due to limited pertinent data, a recent meta-analysis failed to illustrate any significant distinction between the use of non-selective NSAIDs and COX-2 inhibitors.
Another meta-analysis assessed both NSAIDs and myorelaxants, and consistent with the results of this study, both categories of drugs were considered promising in tackling acute LBP. Current guidelines on the pharmacological treatment of acute LBP present diverse recommendations. Specific guidance to pharmacologically manage LBP in acute scenarios was only provided by Qaseem et al. and Oliveira et al., both suggesting the utilization of NSAIDs.
Corp et al.'s examination of European guidelines underscored moderate evidence opposing the utilization of paracetamol and strong evidence against the utilization of most anticonvulsants, antidepressants, and myorelaxants. The evidence for NSAIDs and opioids, however, was deemed inconsistent. Nevertheless, the authors characterized the available evidence as "mixed," and there was a notable paucity of specific indications in the acute setting. The clinical practice recommendations outlined by Oliveira et al. also emphasized the highly diverse nature of recommendations in the literature regarding the pharmacological treatment of acute LBP.
Various classes of drugs received support from some authors while being discouraged by others. Nevertheless, a consensus in favor of using NSAIDs in the context of acute LBP was evident among most authors. When comparing the findings of the current study on acute LBP with the literature on the pharmacological therapy of chronic LBP, a notable disparity in data availability between the two conditions was apparent. The discrepancy was attributed to a higher number of compounds investigated for chronic conditions compared to the acute setting.
Among the medications scrutinized in both scenarios, myorelaxants demonstrated superior efficiency in the acute setting. This observation aligned with the notion that these compounds offered acute pain relief but exhibited little long-term impact on muscle spasms. On the other hand, NSAIDs were found to alleviate pain in both acute and chronic settings. The present study aligned with the consensus that NSAIDs could be utilized efficiently for addressing acute LBP. However, it advocated that alternative approaches, such as combining with paracetamol or using myorelaxants, should also be taken into consideration.
The presence of various drug classes with a favorable influence on the progression of acute LBP provided options in instances of allergies or when a particular compound might be contraindicated due to a patient's comorbidities or interactions with other medications. The significant inclusion of studies, along with the baseline uniformity of the analyzed cohorts and the low risk of bias among the participants, constituted significant strengths of this study.
NSAIDs, NSAIDs combined with paracetamol, and myorelaxants exhibit a favorable efficacy profile in alleviating symptoms and reducing disability in people with acute LBP, prompting the need for future research to explore the impact of various drugs on LBP recurrence.
Journal of Orthopaedic Research
Nonopioid pharmacological management of acute low back pain: A level I of evidence systematic review
Alice Baroncini et al.
Comments (1)