Categories
Change Password!
Reset Password!


To study the efficacy of dupilumab for atopic dermatitis (AD) management in Korean patients
This long term study comprising of Korean patients with atopic dermatitis determined the therapeutic advantage of dupilumab. Also, this study suggested that ‘adherence‘ and ‚‘proactive therapy' can add up to the effectiveness of dupilumab in real-life practice.
To study the efficacy of dupilumab for atopic dermatitis (AD) management in Korean patients
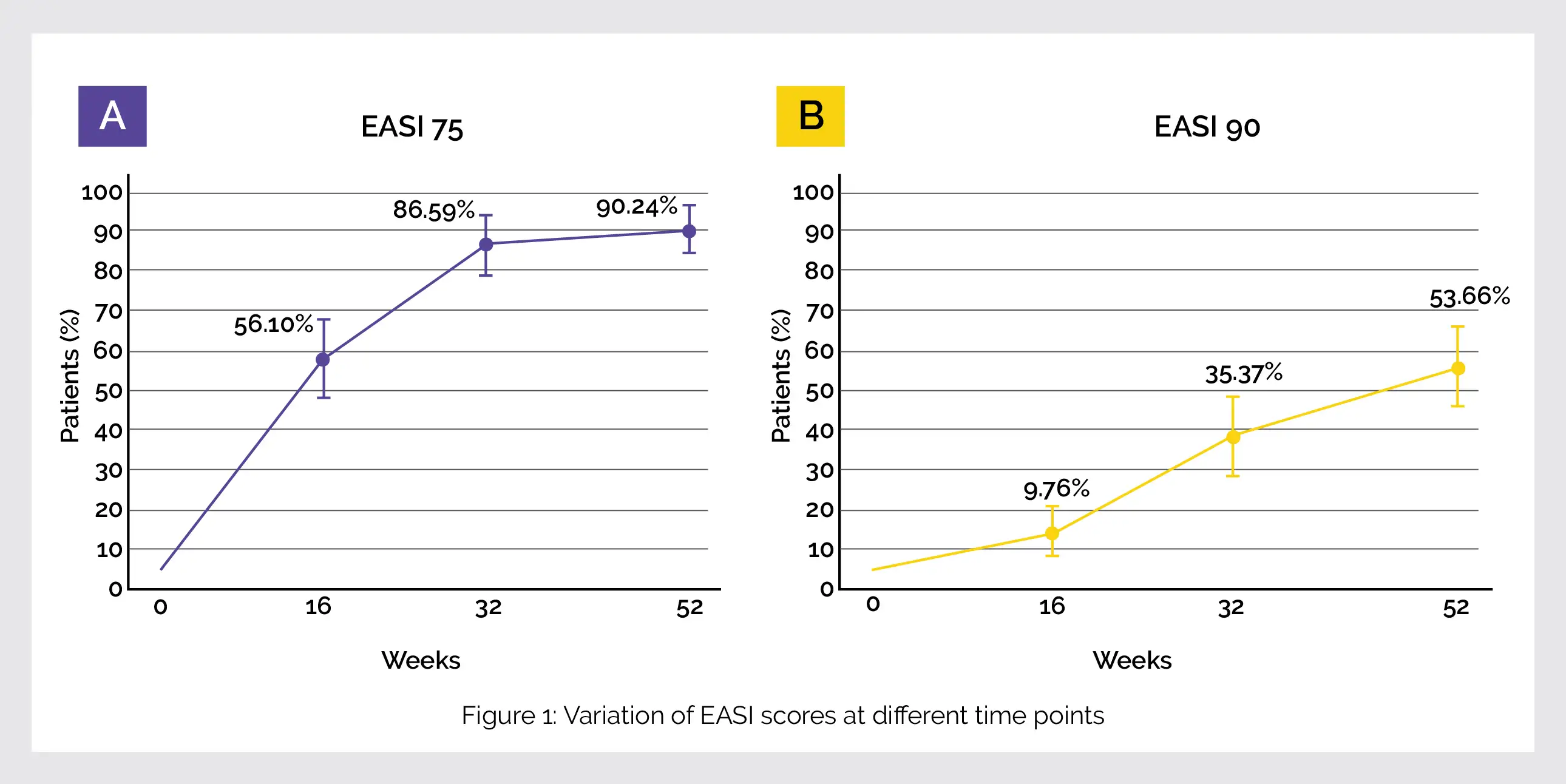
A total of 99 patients with AD were examined. Numerical Rating Scale (NRS) was used to assess pain, Patient-Oriented Eczema Measure (POEM) for atopic eczema, Eczema Area and Severity Index (EASI) and Dermatology Quality of Life Index (DLQI) for quality of life at various durations (starting point, week 16, week 32 and week 52).
A higher improvement at 52 weeks was noted in efficacy outcomes at 52 weeks than at 16 weeks. The EASI (88.1%), peak pruritus NRS (65.6%), POEM (67.2%), and DLQI (69.0%) scores were reduced as compared to the starting point. The percentage of patients achieving EASI 75 and 90 has been shown in the following Figure 1:
Female gender, eosinophilia and raised LDH significantly affected EASI 90. Mild to moderate adverse events (facial erythema and conjunctivitis) were reported.
Dupilumab (monoclonal antibody) can be safely used in patients with AD of moderate to severe intensity.
Scientific Reports
A 52 weeks dupilumab treatment for moderate to severe atopic dermatitis in Korea: long-term efficacy and safety in real world
Dong Hyek Jang et al.
Comments (0)