Categories
Change Password!
Reset Password!


Multicenter, randomized, active-controlled, open-label study to compare the clinical effects of Neurotropin, Limaprost, and their combination for people presenting with lumbar spinal stenosis (LSS) having low back pain (LBP).
For spinal stenosis patients with low back pain, a combination of non-protein extract (Neurotropin) and vasodilator (Limaprost) can have a complementary effect on back and leg pain, and also mend the walking speed.
Multicenter, randomized, active-controlled, open-label study to compare the clinical effects of Neurotropin, Limaprost, and their combination for people presenting with lumbar spinal stenosis (LSS) having low back pain (LBP).
Sixty-four patients with LSS with low back pain were randomly allocated to one of the 3 oral therapies (3 groups), i.e. Neurotropin or N group (20 patients, mean age 76.2 years), Limaprost group or L group (20 patients, mean age 74.4 years) and; Neurotropin/limaprost combination or NL group (24 patients, mean age 71.2 years) for 12 weeks.
Each investigation and observation was done before any drug use and every 2 weeks after its use. Age, gender, height, weight, symptoms duration, intermittent claudication distance, level of spinal stenosis in magnetic resonance imaging, and simultaneous use of painkillers were noted as investigation items. The evaluation items are as under:
The changes were evaluated at each visit (weeks 2 to 12) from baseline value prior to therapy (week 0). The variations were regarded as significant when p < 0.05.
No significant changes in patient characteristics, simultaneous use of painkillers, baseline VAS score, gait balance, or QOL-related scores were found. Considerable improvements in different evaluation items have been shown below (Table 1):
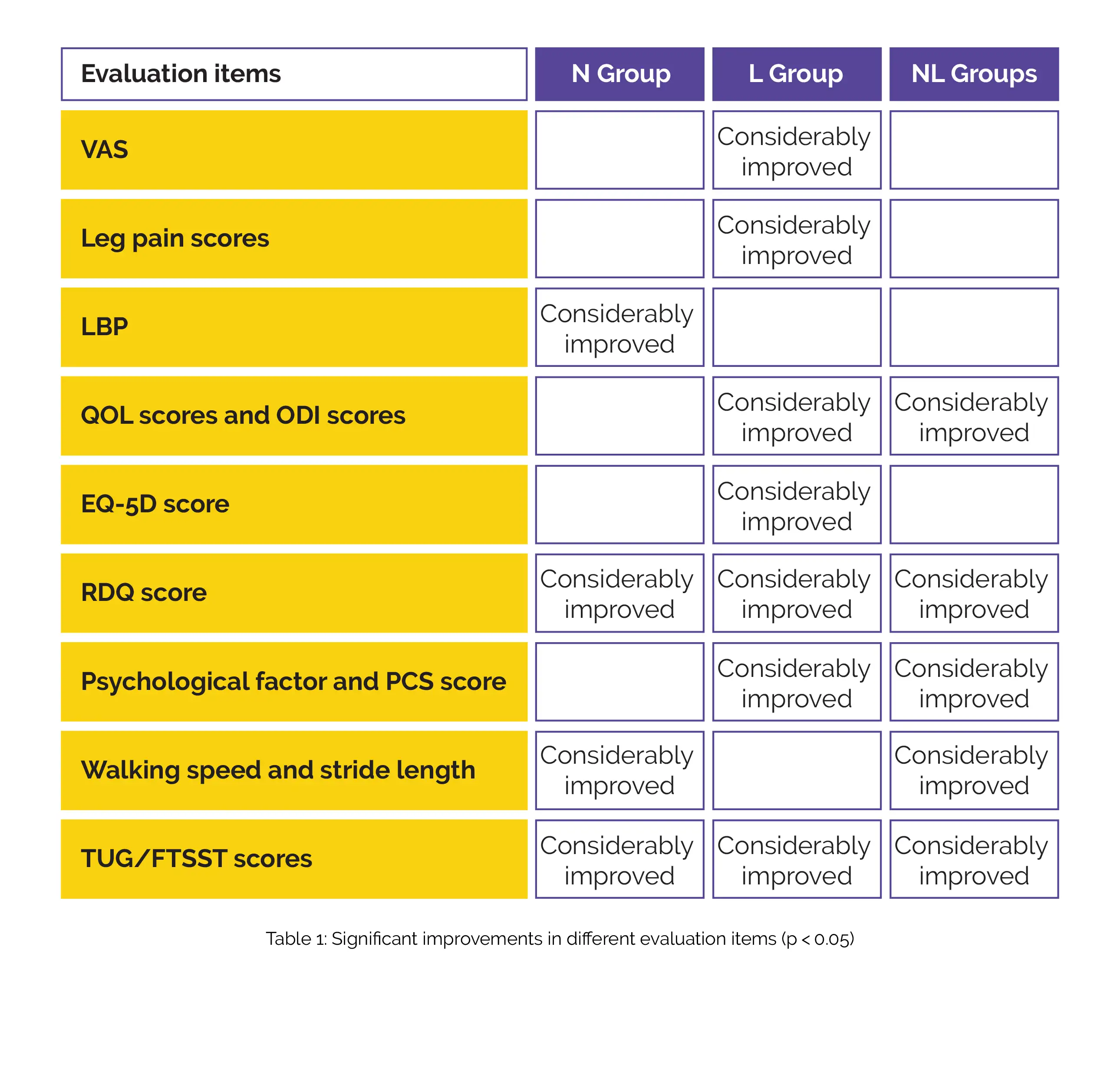
Following 6 and 12 weeks of administration, a substantial improvement in leg pain VAS score was observed in the L group when compared to the NL group, and LBP VAS improved substantially in the N group after 6 weeks than with the NL group. In the NL group, the pace of walking considerably improved after 2 weeks than in the N group and also after 6 weeks than in the L group. After 8 weeks, RMDQ lowered significantly in the L group compared with the NL group.
Neurotropin plus Limaprost is highly effective for recovering the walking speed than used as a monotherapy. The use of Neurotropin can relieve low back pain, improve walking speed/stride length, and also static balance.
Pain and Therapy
Clinical Efficacy of Neurotropin for Lumbar Spinal Stenosis with Low Back Pain
Yawara Eguchi et al.
Comments (1)