Categories
Change Password!
Reset Password!


This randomized, phase 2b, double-blind, placebo-controlled trial was carried out to study the effect of lanifibranor, a pan-peroxisome proliferator-activated receptor (pan-PPAR) agonist, for the management of noncirrhotic, highly active nonalcoholic steatohepatitis (NASH).
In people with nonalcoholic steatohepatitis, lanifibranor (1200-mg
dose) led to a considerable decline of at least 2 points in the SAF-A score
without the aggravation of fibrosis when compared to placebo.
This randomized, phase 2b, double-blind, placebo-controlled trial was carried out to study the effect of lanifibranor, a pan-peroxisome proliferator-activated receptor (pan-PPAR) agonist, for the management of noncirrhotic, highly active nonalcoholic steatohepatitis (NASH).
The study
recruited people suffering from NASH. Participants were randomized to get
lanifibranor (800 mg or 1200 mg) or
placebo once daily for twenty-four weeks. The major outcome was a reduction of
at least two points in the SAF-A score (activity part of Steatosis, Activity,
Fibrosis [SAF] scoring system that includes scores for inflammation and
ballooning, ranges from 0 to 4 with higher scores depicting a more serious
activity of disease) without deterioration of fibrosis. Regression of fibrosis
and NASH resolution were the secondary outcomes.
Overall, 247 people underwent randomization. Notably, 42% of people (103/247) had type 2 diabetes mellitus and 76% (188/247) were found to have fibrosis (moderate or advanced). The proportion of people who had a decrease of at least two points in the SAF-A score without the aggravation of fibrosis was considerably greater among people who were given the 1200-mg dose of lanifibranor when compared to the other two groups.
Both 800-mg and 1200-mg doses of lanifibranor were favored over the placebo for betterment in fibrosis stage of at least 1 without the aggravation of NASH, resolution of NASH + improvement in fibrosis stage of at least 1, and resolution of NASH without the aggravation of fibrosis, as shown in Table 1:
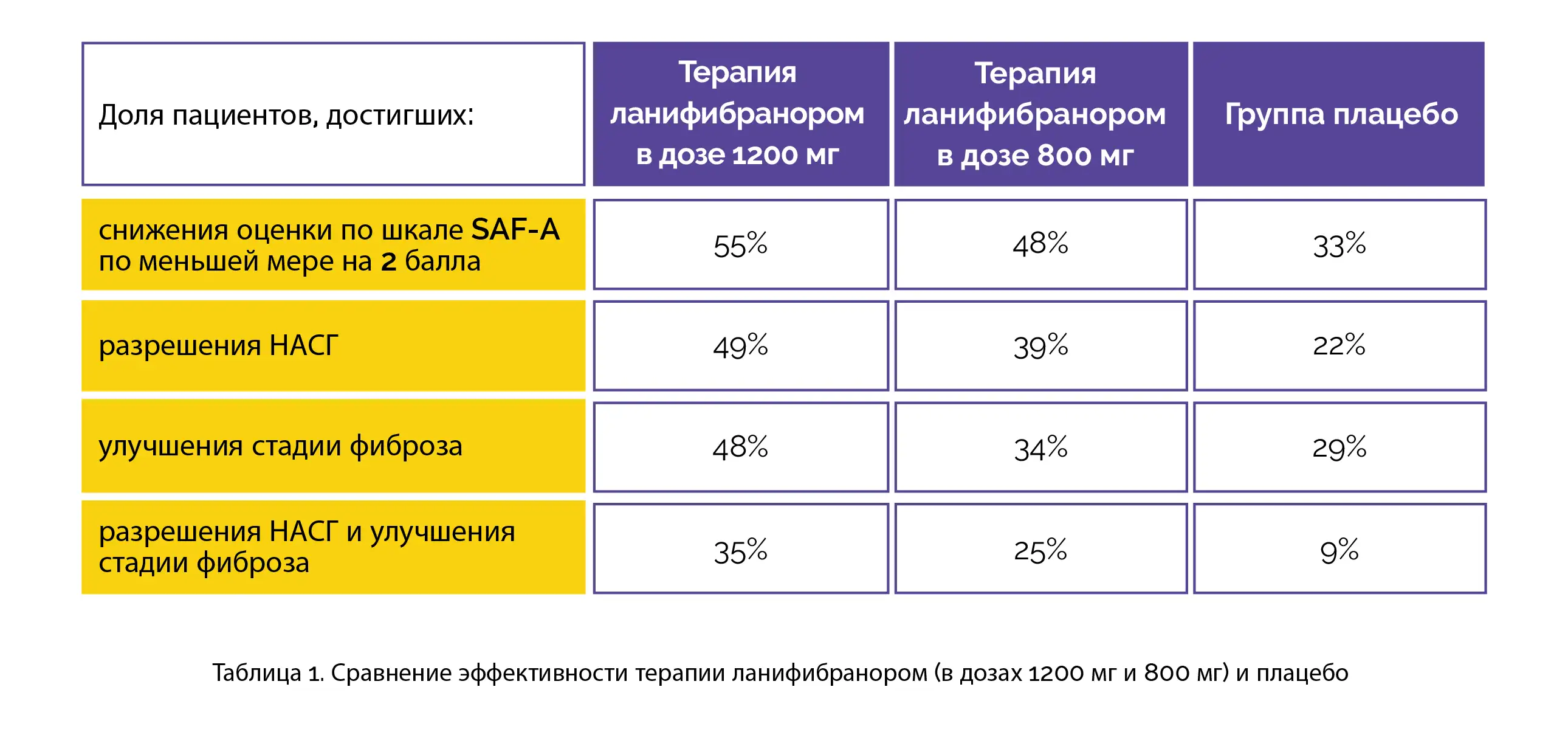
Lanifibranor recipients exhibited reduced levels of liver enzyme and improved levels of the majority of lipid, fibrosis, and inflammatory biomarkers. For noxious events, the dropout rate was less than 5% and was found to be comparable across the trial groups. Weight gain, peripheral edema, nausea, diarrhea, and anemia were found to occur more commonly in lanifibranor-recipients when compared to placebo recipients.
Compared
to placebo recipients, a greater proportion of people receiving a 1200-mg dose
of lanifibranor reported a considerable decline of at least 2 points in the
SAF-A score without deterioration of fibrosis. These results support further
evaluation of lanifibranor in phase III trials.
The New England Journal of Medicine
A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH
Sven M. Francque et al.
Comments (0)