Categories
Change Password!
Reset Password!


Based on responses and changes in severity in patient-reported disability outcomes, a pooled analysis sought to assess Fremanezumab's capacity to lessen migraine-related impairment.
After 12 weeks of therapy, Fremanezumab showed clinically significant improvements in disability scores related to headache and migraine.
Based on responses and changes in severity in patient-reported disability outcomes, a pooled analysis sought to assess Fremanezumab's capacity to lessen migraine-related impairment.
In this study, three double-blind Phase III trials (FOCUS, HALO CM, HALO EM) were included. Subjects with episodic or chronic migraine were randomized to receive Fremanezumab on a quarterly or monthly basis or a matching placebo for a period of 12 weeks. The Migraine Impairment Assessment (MIDAS) and 6-item Headache Impact Test (HIT-6) and questionnaires were used to evaluate migraine-triggered disability.
According to the American Headache Society, a statistically significant decrease in impairment was defined as a reduction of ≥ 5 points on the HIT-6, ≥ 5 points on the MIDAS when the baseline score was between 11 and 20, or a reduction of ≥ 30 points when the baseline score was greater than 20. The percentages of patients who showed changes in severity for each outcome were also assessed.
Fremanezumab substantially outperformed placebo in terms of the percentage of patients whose HIT-6 scores decreased by five points from baseline, as shown in Figure 1:
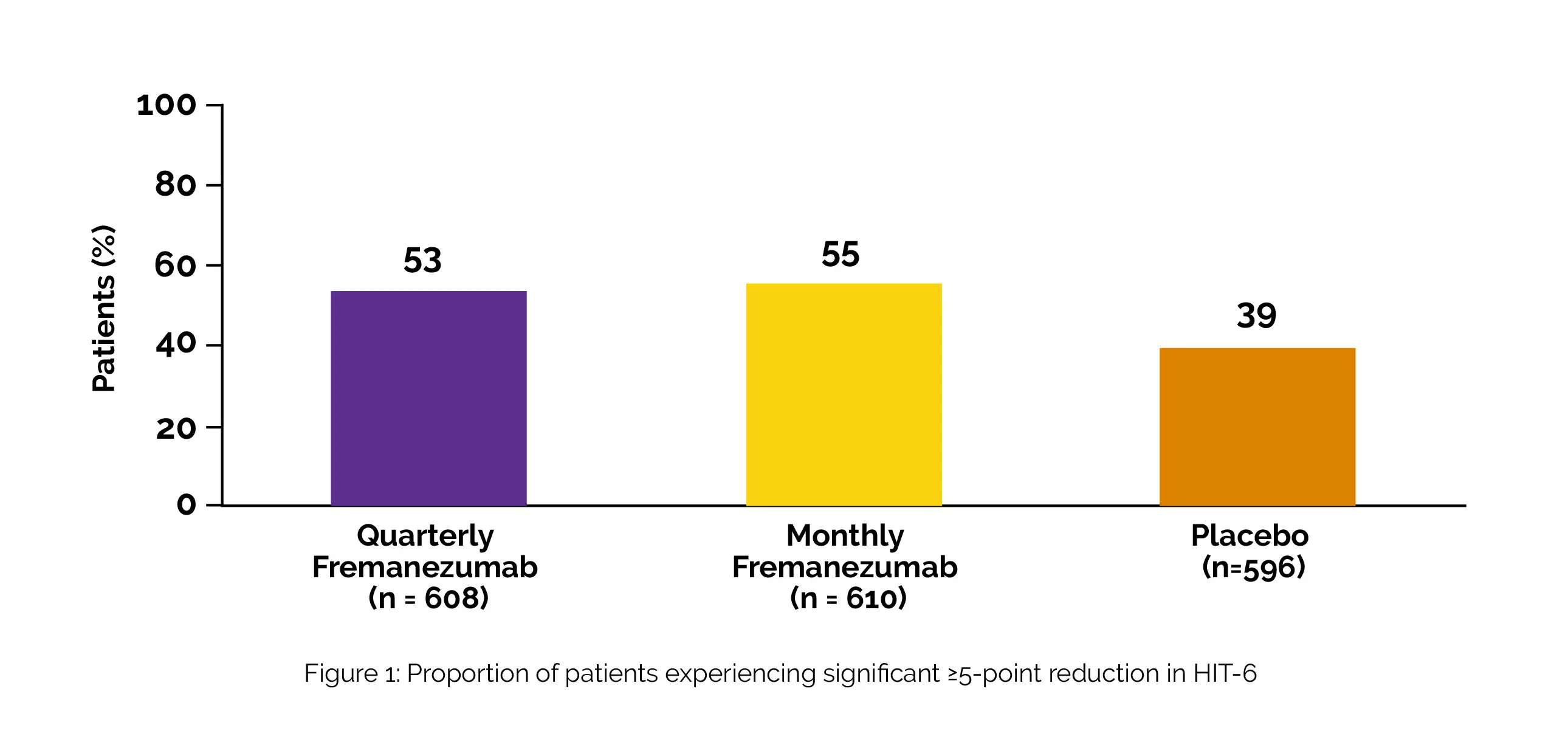
Compared to placebo, Fremanezumab substantially reduced MIDAS scores by five points from the baseline for individuals with baseline scores of 11 to 20 (n = 234). Fremanezumab substantially outperformed the placebo in terms of proportions of patients who achieved ≥ 30% decline in MIDAS scores from baseline for patients with baseline MIDAS scores of > 20 (n = 1266), as shown in Figure 2:
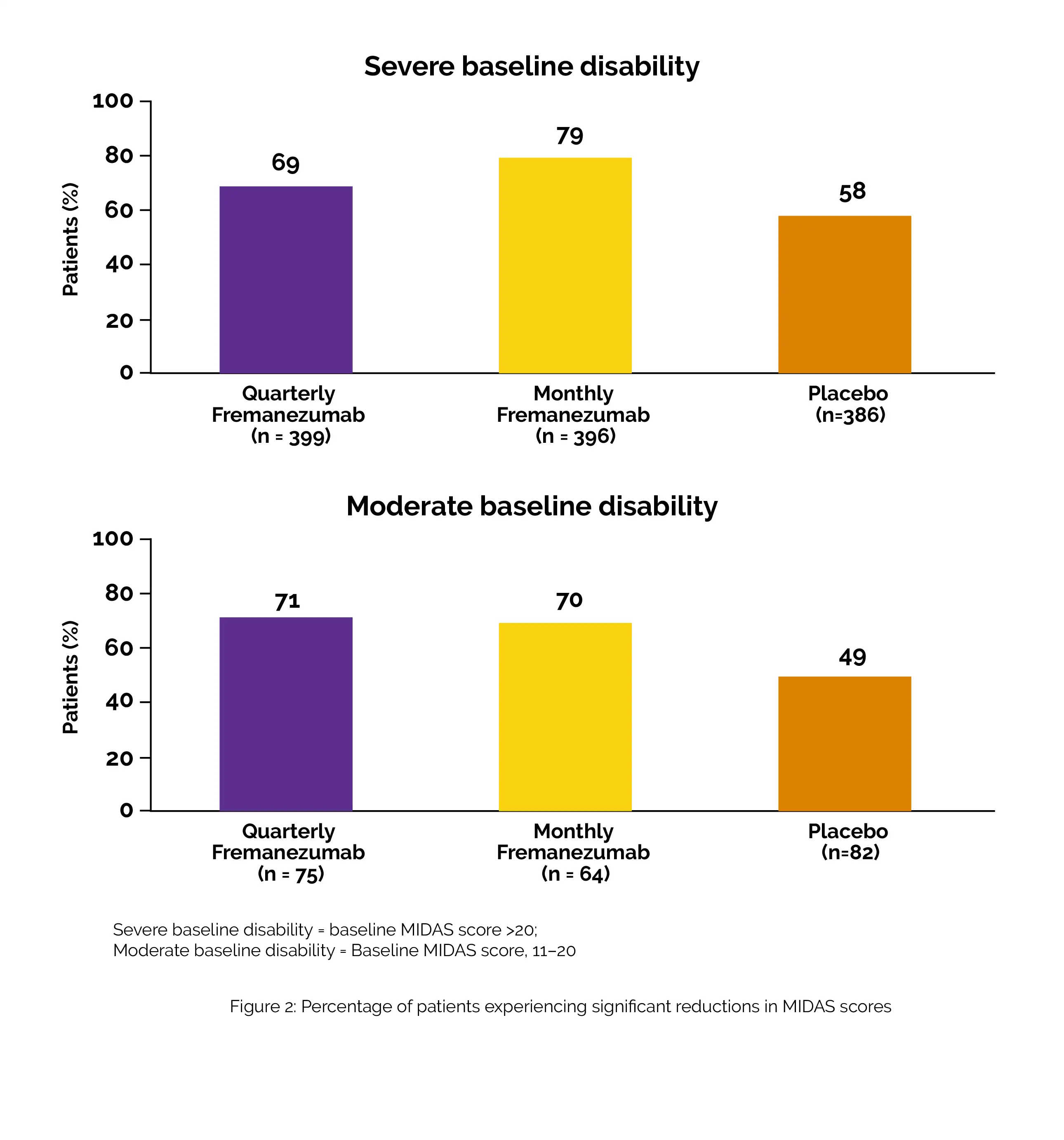
With quarterly and monthly Fremanezumab, the proportions of subjects with changes of 1 to 3 grades lower in HIT-6 or MIDAS disability severity were considerably higher when compared to placebo.
The monoclonal antibody Fremanezumab showed favorable clinical benefits like mitigating migraine symptoms, enhancing patient outcomes, and offering a better quality of life in people with episodic and chronic migraine.
The Journal of Headache and Pain
Impact of Fremanezumab on disability outcomes in patients with episodic and chronic migraine: a pooled analysis of phase 3 studies
Peter McAllister et al.
Comments (0)