Categories
Change Password!
Reset Password!


To assess the impacts of two different dietary restriction approaches compared to medically optimized treatment for individuals diagnosed with IBS.
Dietary interventions could serve as an initial therapeutic approach for people with irritable bowel syndrome (IBS), which can effectively alleviate the severity of IBS symptoms when used along with medical therapies.
To assess the impacts of two different dietary restriction approaches compared to medically optimized treatment for individuals diagnosed with IBS.
The participants, aged 18 years or older, were diagnosed with moderate-to-severe IBS according to Rome IV diagnostic standards, with an IBS Severity Scoring System (IBS-SSS) score of 175 or more, and without other serious conditions or food allergies. They were randomly assigned using web-based randomization into 3 groups: a low FODMAP diet i.e fermentable oligosaccharides, disaccharides, monosaccharides, and polyols combined with standard IBS dietary advice by the UK National Institute for Health and Care Excellence (LFTD diet), a low-carbohydrate diet optimized for fibre, low in total carbohydrates, and rich in protein and fat (referred to as the low-carbohydrate diet), or medical therapy tailored to their predominant IBS symptoms.
The intervention lasted for 4 weeks. After this period, participants in the dietary groups were informed about their respective diets and encouraged to continue for a follow-up of 6 months.
Participants in the LFTD group were enlightened on how to slowly reintroduce the FODMAP diet. Participants receiving medical therapy were suggested diet counselling along with enduring their medication. The primary outcome measure was the proportion of participants who responded to the 4-week intervention (a decrease of 50 points or more in IBS-SSS) compared to the starting of the intervention. This was analyzed based on a modified intention-to-treat approach, including all participants who began the intervention. Assessment of safety was done in the modified intention-to-treat population.
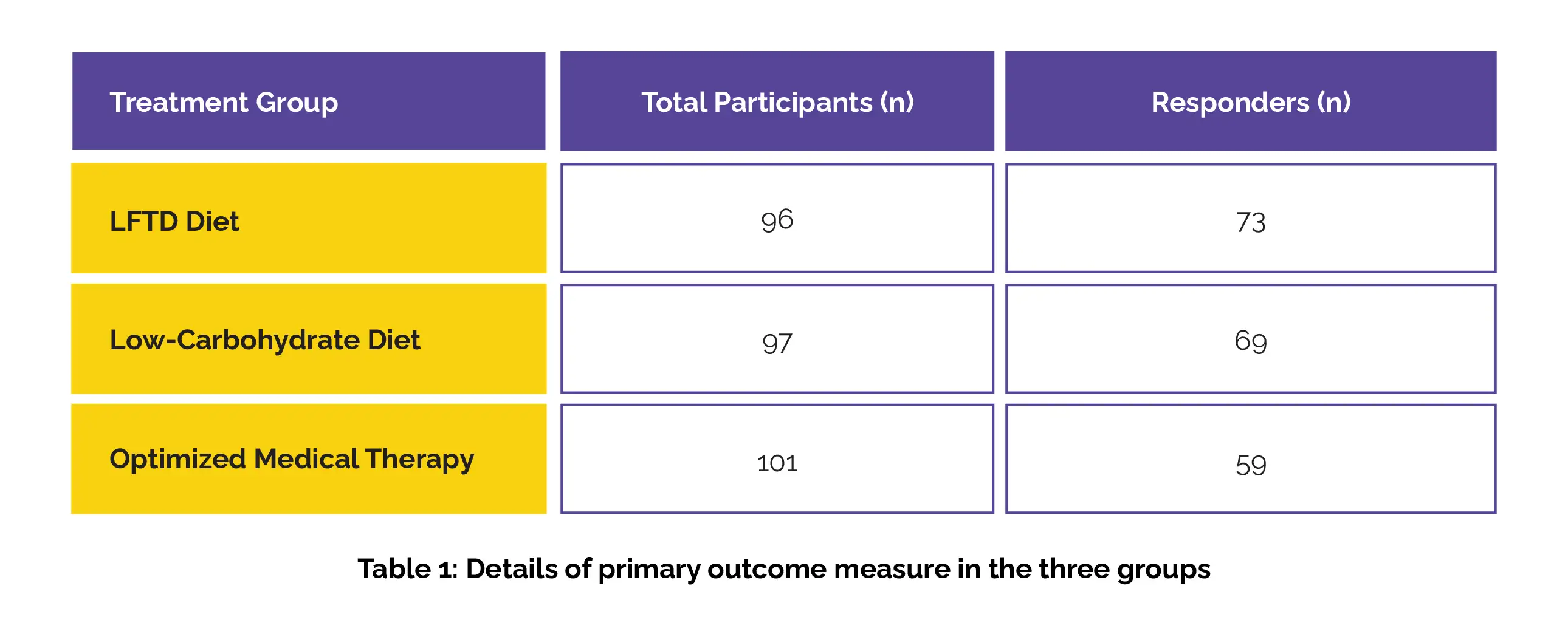
Overall, 1104 individuals were screened for eligibility, out of which 304 were randomly assigned to the study groups. Ten participants were excluded post-randomization, resulting in 294 participants included in the modified intention-to-treat analysis (96 patients in the LFTD diet group, 97 patients in the low-carbohydrate diet group, and 101 patients in the optimized medical therapy group). Among these, 241 (82%) patients were female; mean age of 38 years.
After 4 weeks, a statistically significant decrease of 50 points or more in IBS-SSS was observed in 76% of participants in the LFTD diet group, 71% of participants in the low-carb group, and 58% of participants in the medical therapy group (Table 1):
High completion rates were reported for the 4-week intervention, with 91 (95%) of 96 participants in the LFTD group, 92 (95%) of 97 in the low-carbohydrate group, and 91 (90%) of 101 in the optimized medical therapy group.
Adverse events led to discontinuation of the intervention in 2 individuals from each of the dietary intervention groups. Five of the 91 participants in the medical therapy group withdrew due to the side effects. No severe adverse events or treatment-related fatalities were reported.
The severity of IBS symptoms decreased notably with both 4-week dietary interventions and optimized medical treatment, with a more pronounced impact observed in the dietary intervention groups. Future investigation is required for more personalized treatment approaches.
The Lancet Gastroenterology & Hepatology
A low FODMAP diet plus traditional dietary advice versus a low-carbohydrate diet versus pharmacological treatment in irritable bowel syndrome (CARBIS): a single-centre, single-blind, randomised controlled trial
Sanna Nybacka et al.
Comments (0)