Categories
Change Password!
Reset Password!


Safety and effectiveness of colloidal bismuth pectin (CBP) quadruple therapy and bismuth potassium citrate (BPC) quadruple therapy were contrasted for Helicobacter pylori (H. pylori) elimination in a multicenter, non-inferiority, double-blind, randomized clinical trial.
Both colloidal bismuth pectin and bismuth potassium citrate quadruple therapy are effective, safe and have good compliance in H. pylori management.
Safety and effectiveness of colloidal bismuth pectin (CBP) quadruple therapy and bismuth potassium citrate (BPC) quadruple therapy were contrasted for Helicobacter pylori (H. pylori) elimination in a multicenter, non-inferiority, double-blind, randomized clinical trial.
People with H. pylori infection and no history of elimination received one of four treatments for 14 days: 1 g Amoxicillin two times a day, 500 mg Tetracycline thrice a day, 20 mg Esomeprazole two times a day plus 200 mg CBP thrice a day, or BPC 240 mg two times a day. Minimum four weeks following therapy, the elimination rate was assessed by 13C-urea breath tests.
Out of 406 patients, 339 individuals were randomized. According to intention-to-treat analysis, the cure rates (primary outcome) of CBP and BPC quadruple therapy is depicted in Table 1:
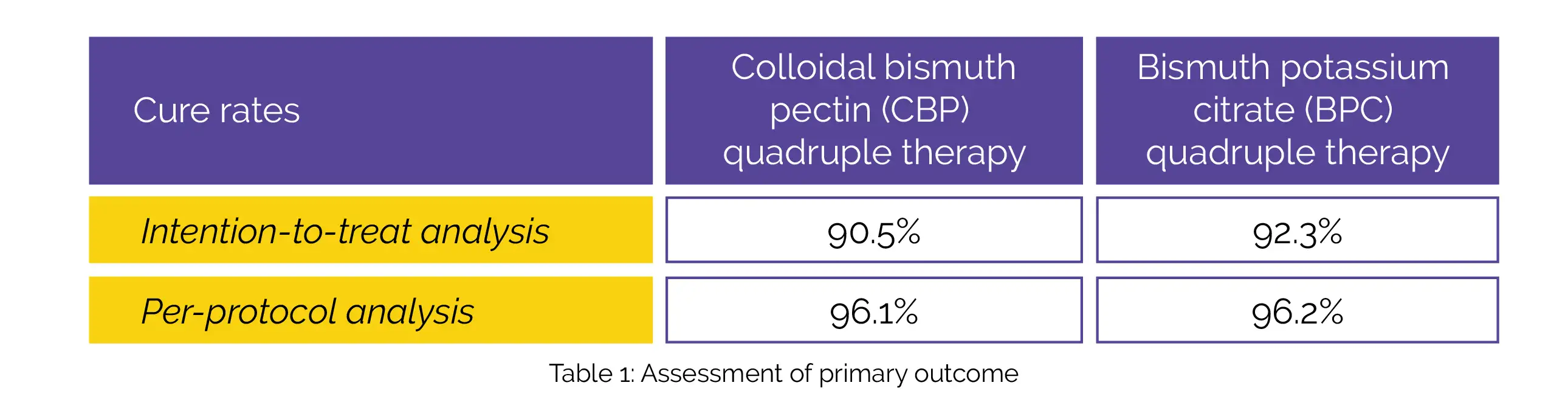
In the intention-to-treat and per-protocol analyses, CBP quadruple therapy exhibited non-inferiority to BPC quadruple therapy. There were no differences between both groups in the frequency of adverse events and compliance.
In the first-line treatment of H. pylori, both CBP and BPC quadruple therapy for 14 days offered high effectiveness, good compliance and had a favorable safety profile.
Helicobacter
Colloidal bismuth pectin-containing quadruple therapy as the first-line treatment of Helicobacter pylori infection: A multicenter, randomized, double-blind, non-inferiority clinical trial
Yong Xie et al.
Comments (0)