Categories
Change Password!
Reset Password!


The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that belongs to the betacoronavirus genus, led to the coronavirus disease 2019 (COVID-19) outbreak.
Treatment with methylprednisolone was related to better clinical outcomes and reduced short-term mortality, without elevating risk of secondary infections in COVID-19 patients.
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that belongs to the betacoronavirus genus, led to the coronavirus disease 2019 (COVID-19) outbreak. As of 6 September 2021, about 221 countries and territories were impacted by COVID-19, and around 220,563,227 cases have been reported around the world, out of which 4,565,483 people have died. This triggered a huge threat to human society. Nearly 5% of people suffering from COVID-19 need to be transferred to the intensive care unit (ICU) for respiratory support and are linked with a high mortality rate.
Numerous studies have demonstrated that the rapid clinical deterioration of coronavirus-infected people is closely associated with cytokine storm/hyper-inflammation. When a cytokine storm comes up, the T cells, macrophages and natural killer cells hyper-activate and multiply rapidly. Massive inflammatory cytokines are liberated that leads to the apoptosis of pulmonary endothelial and epithelium cells, damage to the alveolar epithelial cell barrier and pulmonary microvascular.
This stimulates hypoxia, alveolar edema, vascular leakage, and ultimately acute respiratory distress syndrome (ARDS), that is the major reason for death in COVID-19 patients. Thereby, apart from antiviral treatment, hyper-inflammatory response inhibition and tissue damage prevention are also the emphasis of coronavirus disease treatment. Glucocorticoids (anti-inflammatory agents) have been utilized formerly in respiratory diseases like ARDS, severe bacterial pneumonia, chronic obstructive pulmonary disease and asthma.
The World Health Organization (WHO) warned against systematic utilization of corticosteroids in patients with coronavirus infection at the beginning of the COVID-19 pandemic. Following the publication of the RECOVERY trial, WHO suggested employing corticosteroids in SARS-CoV-2 infected patients. In the RECOVERY trial, glucocorticoids (dexamethasone) could profoundly alleviate fatality in severe COVID-19 patients, particularly in people receiving mechanical ventilation support, when compared to standard care without corticosteroids.
A recent systematic review examined data from seven randomized controlled trials (RCTs) to determine the effectiveness of glucocorticoids in 1703 critically-ill people suffering from coronavirus disease. When compared with placebo, the findings revealed that the use of corticosteroids was related to reduced 28-day all-cause mortality. It is well-documented that methylprednisolone is the preferred glucocorticoid to treat ARDS in ICU instead of dexamethasone.
RATIONALE BEHIND RESEARCH
Since the commencement of COVID-19 outbreak, there have been numerous studies on the treatment of COVID-19 with methylprednisolone. However, the findings are not consistent, thus this systematic review and meta-analysis were carried out.
OBJECTIVE
The aim of this systematic review and meta-analysis was to assess the safety and efficacy of methylprednisolone use in COVID-19 infected people compared with no corticosteroids therapy.
Literature search
Utilizing various electronic databases such as Web of Science, Cochrane Central Register of Controlled Trials and PubMed, the literature search was performed. The search strategy utilized in PubMed and changes based on the rules of every database was (COVID-19) AND (((corticosteroids) OR (“methylprednisolone” [Mesh])) OR (methylprednisolone)).
During literature search, English was set as a language restriction and for additional publications, the reference list of retrieved papers was also screened.
Inclusion criteria
Following studies were included in this systematic review:
Exclusion criteria
Following studies were excluded:
Study selection and Data extraction
Recording of the following information from each eligible study was done: data required for quality evaluation, major endpoint and other study features, methylprednisolone prescription, population characteristics, study interval, study design, study location, and the first author’s name. Population characteristics incorporated mean age and sample size of both arms, along with the disease status of the patients enrolled in each study.
The total number of volunteers and number of events of each group were extracted for dichotomous variables. To extract continuous variables, standard deviation and mean were utilized. The standard deviation was calculated via statistical algorithms if the studies stated continuous data like range and/or median values.
Data and Statistical Analysis
With the utilization of Review Manager (version 5.1.0), statistical analysis was performed. For dichotomous data, the risk ratio (RR) for every study was grouped to acquire pooled RR with a corresponding 95% confidence interval (CI). Continuous variables were analyzed by calculating mean difference (MD) with a corresponding 95% CI. All the findings were examined for statistical and clinical heterogeneity.
Since the clinical heterogeneity was unavoidable, the subgroup analysis was carried out based on the disease severity, the dosage and course of methylprednisolone therapy, and various types of study design. The standard body weight of the adult was set at 60 kg, the high-dose methylprednisolone was defined as > 2 mg/ kg/day, and the low-dose was defined as ≤ 2 mg/kg/day. Classification of the methylprednisolone therapeutic course was done as ≤ 3 days, ≤ 7 days and > 7 days.
The participants' severity was categorized as non-severe and severe because of the distinct definitions in each incorporated study. Assessment of the statistical heterogeneity among various studies was performed using Cochran’s Q test in which p < 0.1 depicts the existence of significant heterogeneity. In case of significant statistical heterogeneity, the random-effects model would be utilized. Furthermore, sensitivity analysis would be applied to determine the result's stability. Else, the fixed-effect model will be selected. For evaluating statistical heterogeneity, a forest plot was used.
Risk of Bias and Quality assessment
On the basis of criteria given by Cochrane Collaboration, the quality assessment of RCT was done. In this, the risk of bias of each trial is classified as unclear risk, high risk or low risk. For quality assessment of non-RCTs, the methodological index for non-RCT studies (MINORS) was utilized.
Evaluation of publication bias was done via formation of a funnel plot with visual determination of asymmetry. For quantitatively assessing the publication bias (STATA 12.0), the Egger’s regression was utilized. A p value<0.05 was deemed statistically relevant.
Study outcomes
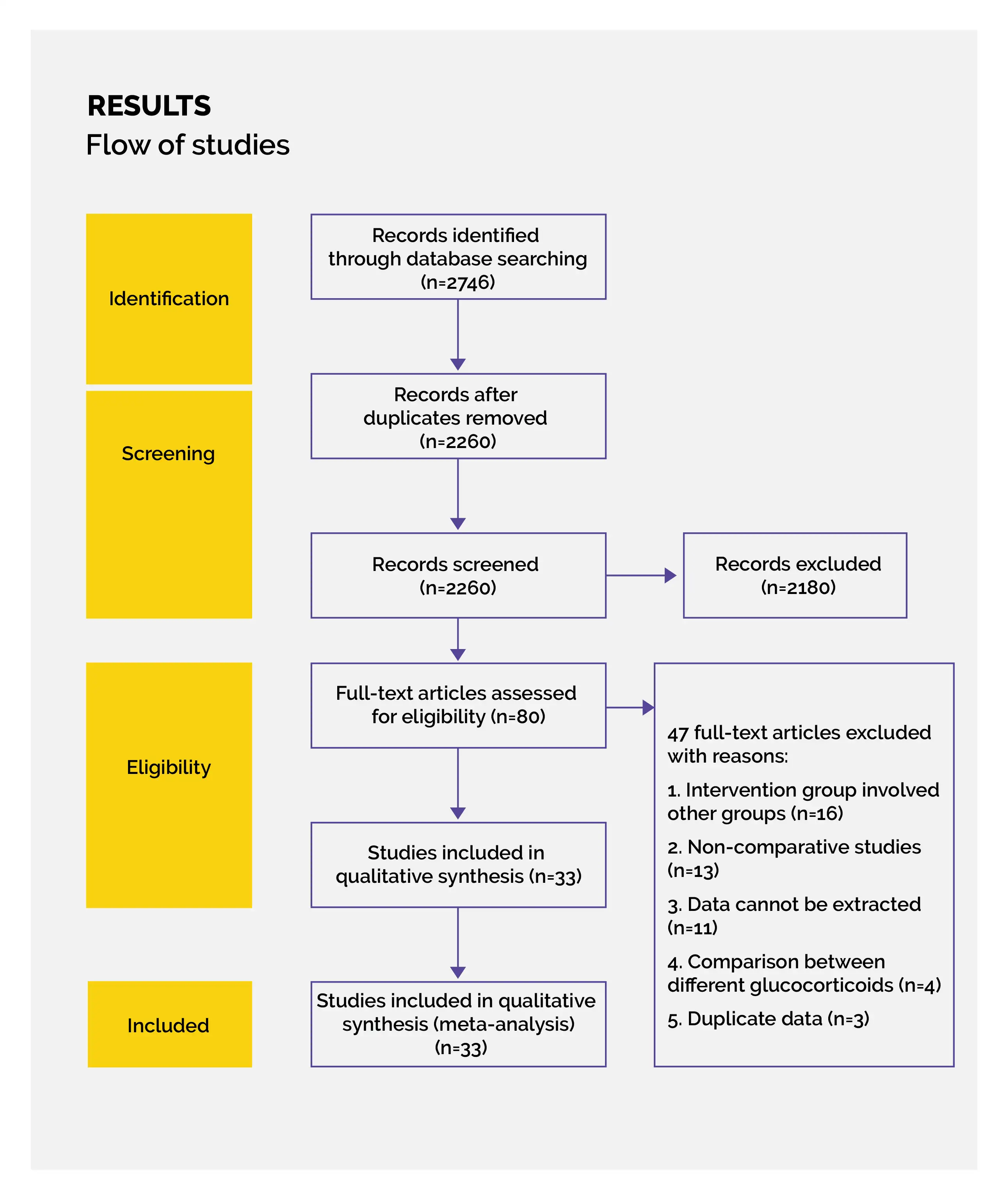
Outcomes
Study and participant characteristics:
Study quality:
Effect of intervention on the outcome:
The findings of the present study demonstrated that compared with no glucocorticoids therapy, methylprednisolone for the treatment of COVID-19 was associated with less requirement for mechanical ventilation and ICU admission, reduction in short-term mortality, and elevation in 28-day ventilator-free days, without raising the risk of secondary infections. However, it might prolong the viral shedding period.
The subgroup assessment illustrated that short-term low-dose methylprednisolone therapy (1–2 mg/kg/day for ≤ 7 days) in severe SARS-CoV-2 infected people was linked with comparatively improved clinical outcomes, without elevating the viral shedding duration, when compared to standard care without corticosteroids. Glucocorticoids can play a favorable role in treating COVID-19 pneumonia.
The RECOVERY trial cited in WHO guidelines substantiated the benefits of glucocorticoids for treating severe COVID-19. However, the drug used was dexamethasone. But, it was stated that the response of methylprednisolone was more than that of dexamethasone in vitro. Draghici et al. investigated an initial characterization of the major pro-inflammatory pathways elicited by COVID-19 infection on human lung epithelial cells. Methylprednisolone was recognized as the most effective agent that targets pivotal components of the inflammatory pathway implicated in ARDS.
Additionally, the findings of their study proposed that methylprednisolone would revert the largest number of the gene disturbed by coronavirus disease, followed by dexamethasone. Moreover, they proved the effectiveness of methylprednisolone in a clinical trial in which 30-day all-cause mortality occurred at a considerably reduced rate in the methylprednisolone arm than conventional therapy arm.
In a retrospective cohort study carried out by Fernandez-Cruz et al., the survival of people with COVID-19 pneumonia was greater in methylprednisolone-treated people vs. people not treated with methylprednisolone.
In a randomized study, Hamed et al. demonstrated that people with SARS-CoV-2 infection when given methylprednisolone exhibited lower ICU admission and ventilation rates, and a decline in 45-day mortality when compared to standard care recipients. This meta-analysis also depicted the advantages of methylprednisolone therapy in people having COVID-19 pneumonia, particularly in severe cases. Theoretically, glucocorticoids can show their anti-inflammatory effect via non-genetic and genetic pathways.
Methylprednisolone therapy led to a remarkable reduction in the plasma inflammatory indicators (like IL-6 and CRP) of SARS-CoV-2 infected people. The decrease in the inflammatory response may elicit rapid mitigation of lung injury, better symptom relief and reduced need for mechanical ventilation and ICU admission. This assumption is assisted by the findings of the study. The optimum methylprednisolone prescription to manage COVID-19 people is inconclusive.
In a study by Edalatifard et al., 68 people suffering from severe COVID-19 were randomly allocated get either standard care or methylprednisolone pulse (250 mg/day for 3 days). Reduced hospital mortality and an increased percentage of improved patients were witnessed in the methylprednisolone pulse group. Although, the retrospective cohort study carried out by Fernandez-Cruz et al stated that hospital mortality was not different between pulse treatment and low-dose (1 mg/kg/ day) methylprednisolone treatment.
The subgroup assessment of the study showed that low- and high-doses of methylprednisolone illustrated remarkable favorable effects on mortality benefit and occurrence of secondary infections. Low-dose methylprednisolone (reduced drug costs) can lower the mechanical ventilation and ICU admission risks, even though it may raise the virus clearance time by one day. This might imply that the drug cost savings can aid patients evade suffering from mechanical ventilation and additional hospitalization costs because of ICU stay.
The advantages of these clinical outcomes far outweigh the chance of slightly extending SARS-CoV-2 clearance time. In people diagnosed with severe coronavirus disease, methylprednisolone use did not delay SARS-CoV-2 clearance. Methylprednisolone treatment within one week may be advantageous for people infected with SARS-CoV-2, that is close to the therapeutic course (3–5 days) suggested by the guideline from China. This meta-analysis concentrated on methylprednisolone, that is usually utilized by clinicians for the management of pulmonary inflammatory diseases.
For minimizing the influence of confounding factors on the findings, only those studies were taken into consideration in which methylprednisolone was designed as the sole intervention agent. A recently published meta-analysis was similar to this study and incorporated only five RCTs with a comparatively limited outcome (all-cause mortality) and a small sample size.
In this systematic review, 31 studies were investigated, with a larger sample size, a reduced probability of false negative results and more interesting outcomes assessed, thus leading to more reliable conclusions. With a better understanding of disease and standardization of therapy, more high-quality and robust clinical studies are warranted for further verifying methylprednisolone's superiority to treat people having coronavirus disease.
Clinicians should consider methylprednisolone treatment in people suffering from COVID-19, as it is associated with decreased short-term mortality, less requirement for ICU admission and mechanical ventilation, and a rise in 28-day ventilator-free days.
Steroids
The roles of methylprednisolone treatment in patients with COVID-19: A systematic review and meta-analysis
Shukun Hong et al.
Comments (0)