Categories
Change Password!
Reset Password!


In the course of fetal development, the brain experiences significant and rapid growth.
Raised maternal preintervention 25(OH)D levels were linked to reduced autism risk, lower autistic symptoms, and a minimized likelihood of ADHD diagnosis. But, administering high-dose vitamin D3 during pregnancy did not exhibit any vital influence on autism or ADHD risk in offspring.
In the course of fetal development, the brain experiences significant and rapid growth. Exposures to environmental factors during this critical phase can have enduring implications, potentially influencing the likelihood of developing prevalent neurodevelopmental disorders like autism and attention deficit hyperactivity disorder (ADHD) in the long term. Globally, the prevalence of vitamin D insufficiency during pregnancy has been estimated to exceed 50% among pregnant women. Studies in animals have displayed the importance of vitamin D in the developing brain, impacting functions such as the modulation of neurotransmission and providing neuroprotection.
Given that the fetus depends on the transfer of vitamin D from the mother through the placenta, maternal deficiency in vitamin D has the potential to impact the brain development of the fetus. Past observational investigations have documented a link between maternal vitamin D inadequacy during pregnancy and the heightened risk of ADHD and autism in offspring. Nevertheless, the findings are inconclusive and may be influenced by factors such as season, lifestyle, and diet, introducing potential confounding variables. Additionally, certain studies indicate that reduced gestational vitamin D levels are linked to heightened severity of traits and symptoms associated with autism and ADHD during childhood, although conflicting results exist in other research.
RATIONALE BEHIND RESEARCH
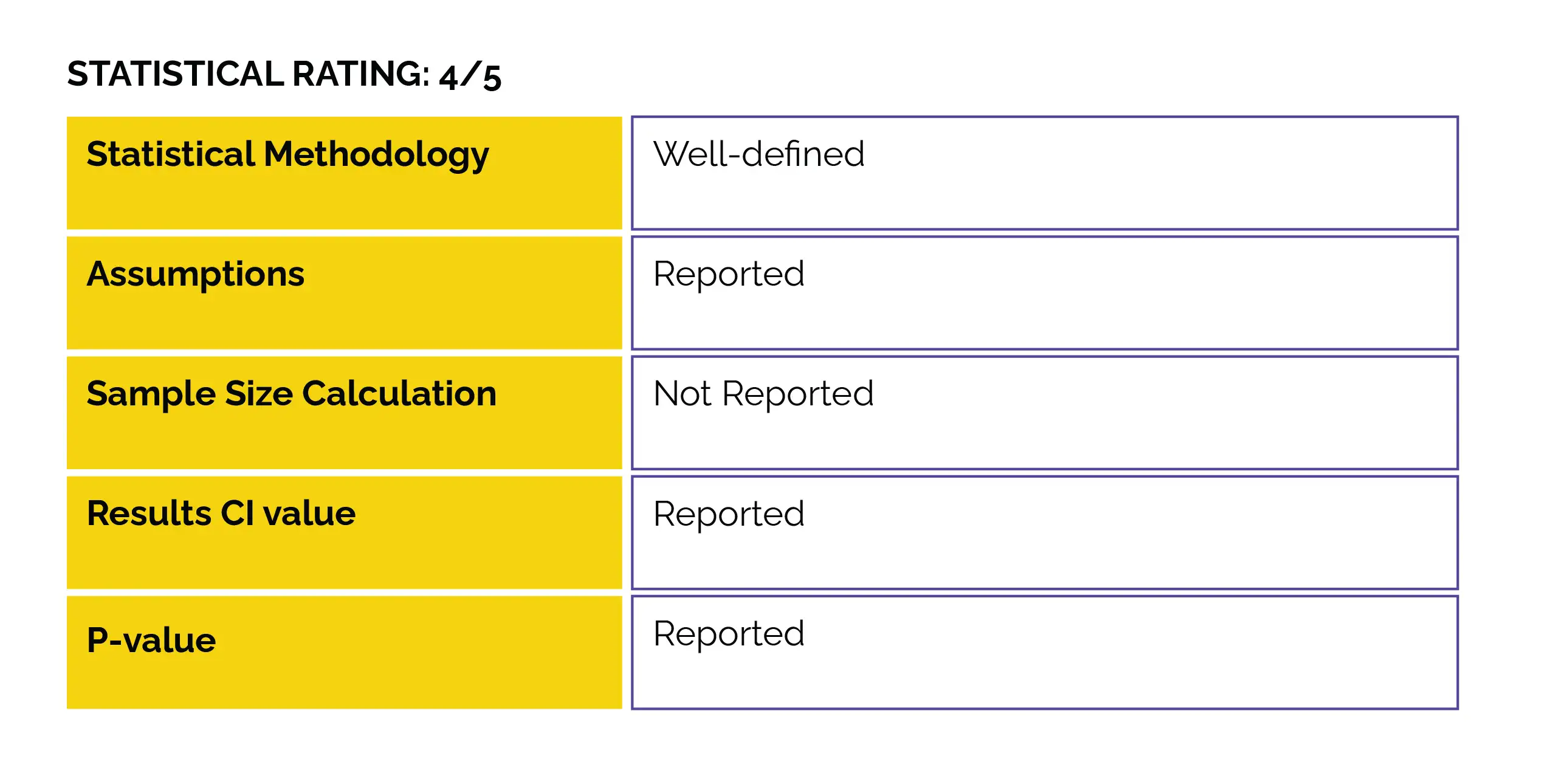
No randomized clinical trials (RCTs) assessing the influence of vitamin D supplementation during pregnancy on neurodevelopmental ailments have been conducted.
OBJECTIVE
This RCT was conducted to determine the impact of high-dose versus standard-dose vitamin D3 use during pregnancy's third trimester. The study aimed to assess autism and ADHD risk, along with the clinically evaluated symptom load at the age of 10, as part of the COpenhagen Prospective Study on Neuro-PSYCHiatric Development (COPSYCH) project.
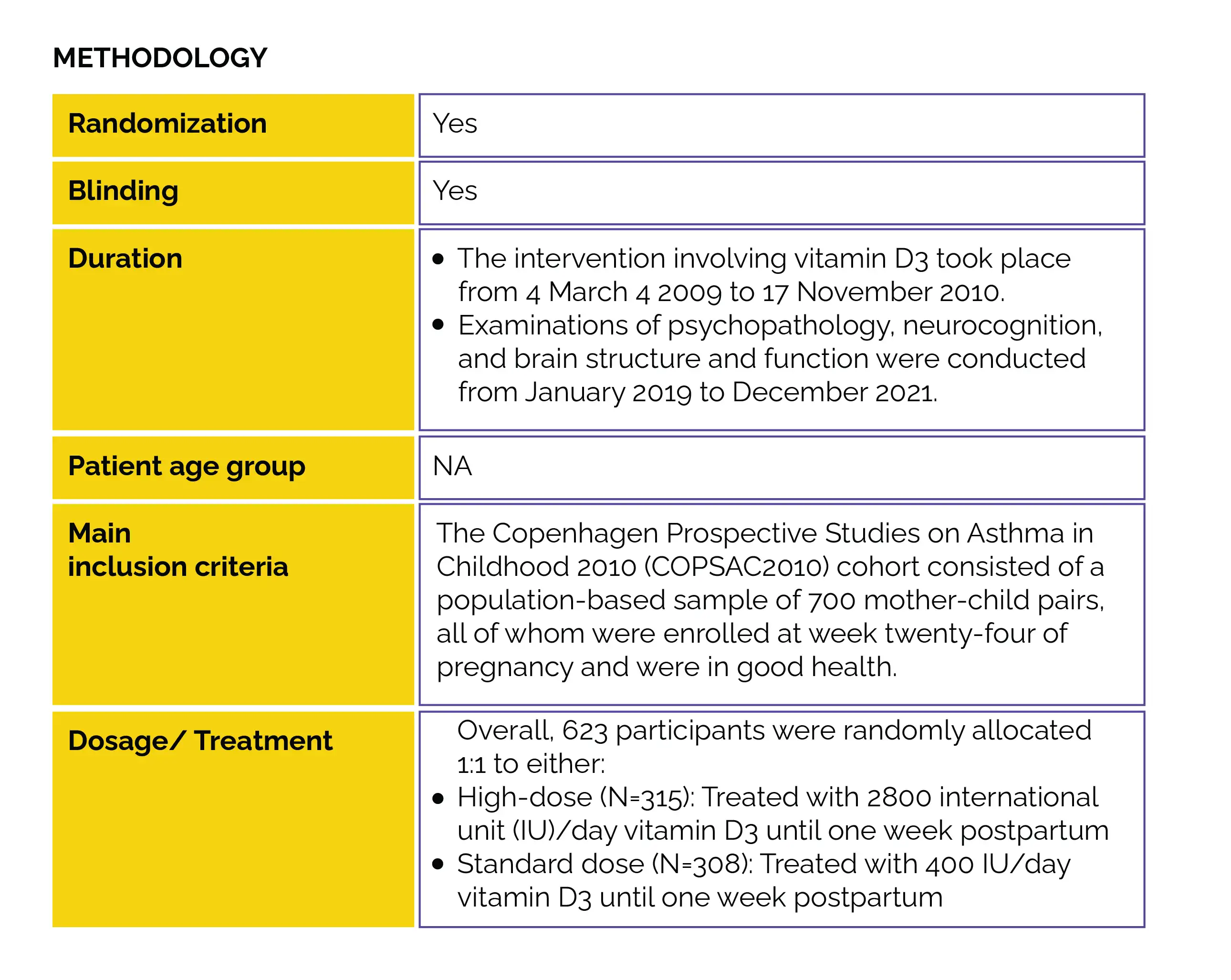
Study outcomes
Outcomes
Baseline: There were no vital differences reported at baseline.
Study outcomes
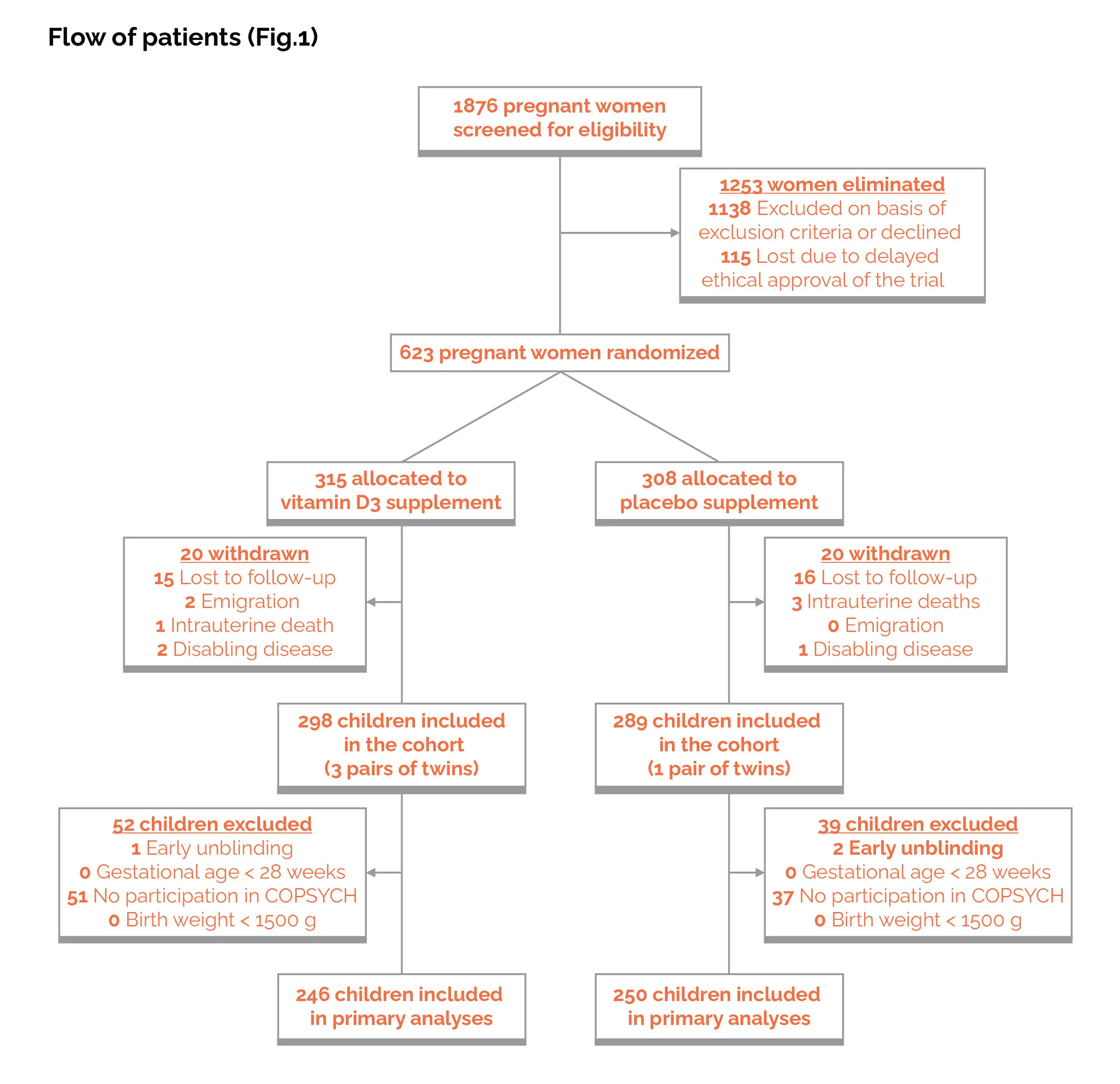
In pregnancy, vitamin D deficiency is connected with the risk of autism and ADHD. This study was part of the COPYCH project nested within the COPSAC2010 cohort and comprised a population-based sample of 700 healthy mother-child pairs recruited at week 24 of pregnancy. The impact of administering a high dose (2800 IU/day) versus a standard dose (400 IU/d) of vitamin D3 from the 24th week of pregnancy until one week after childbirth was examined. Autism and ADHD diagnoses, along with the corresponding symptom severity, were determined utilizing the Kiddie-Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version at the age of 10.
No overall protective effect was observed regarding the risk of symptom severity, ADHD, or autism. However, elevated levels of maternal 25-hydroxyvitamin D [25(OH)D] prior to intervention were correlated with a reduced risk of autism, lower autistic symptom burden, and a diminished likelihood of ADHD. The current study addressed significant drawbacks present in the prior observational research examining the link between gestational serum 25(OH)D levels and the risk of autism and ADHD in the offsprings. The RCT design enabled an impartial exploration of the impact of vitamin D3 use during the crucial phase of late pregnancy, marked by rapid brain growth and neuronal development.
This holds particular significance given the numerous lifestyle factors known to impact vitamin D serum levels. Moreover, this investigation relied on comprehensive clinical assessments conducted by consistently trained nurses and medical doctors at the COPSAC research unit, in contrast to outcomes reported by parents or derived from registries, which are more susceptible to bias. Finally, the continuous follow-up and longitudinal in-depth characterization of the COPSAC2010 cohort enabled the examination of serum 25(OH)D effects from prenatal stages through childhood while accounting for potential confounding variables.
Irrespective of the intervention and considering the potential influence of lifestyle factors, the researchers noted that elevated maternal 25(OH)D levels before the intervention were linked to diminished chances of ADHD and autism. Additionally, there was a lower clinically assessed symptom burden for autism, though not for ADHD. However, when examining parent-reported autistic traits, the connection with autistic symptoms did not reach statistical significance. Prior research has also established a connection between higher maternal serum 25(OH)D during pregnancy and a reduced risk of both ADHD and autism diagnoses. According to the findings of the Generation R study, higher maternal gestational 25(OH)D levels were linked to lower parent-rated autistic trait severity in a cohort of 2866 mother-child pairs.
In a smaller case-control study, elevated gestational 25(OH)D was associated with fewer autistic symptoms estimated by medical care personnel using the Childhood Autism Rating Scale. However, a large birth cohort study reported no connection between gestational 25(OH)D levels and later parent-reported symptoms of ADHD. The overall defense against child autism and ADHD diagnosis or symptom load was not demonstrated by the high-dose vitamin D3 supplementation during pregnancy. The impact of vitamin D usage on the risk of autism or ADHD during pregnancy has not been explored in any previous RCT.
In a prospective analysis conducted in 2016, the autism recurrence rate in newborn siblings dropped from twenty percent to five percent when mothers of kids with autism received vitamin D supplements during pregnancy. These mothers were given 5000 IU of vitamin D3 every day, in contrast to the 2800 IU/day in the current study. The observed discrepancy with the results may, to some extent, be explained by the lower intervention dose of vitamin D3 in the current RCT. In mothers having a serum 25(OH)D level of ≥75 nmol/L during early pregnancy, the high-dose vitamin D3 intervention appeared to ameliorate autism diagnosis risk. This portrays the potential significance of elevated 25(OH)D levels in early pregnancy for typical brain development.
This observation aligned with a substantial registry-based study that demonstrated a connection between lower maternal serum 25(OH)D levels in the first and early second trimesters of pregnancy and a heightened risk of autism diagnosis. Additionally, the early stages of pregnancy are considered vulnerable, as the foundational elements of the neural system are established during the embryonic phase. Therefore, it's plausible that the vitamin D3 intervention in the current study was introduced too late in pregnancy or that the intervention dose was insufficient to yield an effect among mothers with vitamin D deficiency at randomization. A threshold analysis of the effect of vitamin D3 on autistic symptom load according to maternal preintervention serum 25(OH)D was performed.
A U-shaped association was indicative of a protective effect of vitamin D3 intervention among mothers with preintervention levels between ∼55 and 110 nmol/L revealed by the analysis. U-shaped link between vitamin D and bone health, as well as aeroallergen sensitization, have been described previously. Furthermore, a higher risk of schizophrenia among children with either low or high 25(OH)D measured in neonatal dried blood samples has also been reported in a case-control study. Potential non-linear associations should be taken into consideration in future studies on vitamin D and autism. Two prior studies identified sex-specific differences in the connection between early-life vitamin D status and autism. One study found a protective association with higher neonatal vitamin D exclusively in girls, while another study reported a protective association with higher gestational vitamin D in boys and an opposite association in girls.
In the current study, no interaction was witnessed between sex and the intervention for either autism or ADHD. The purported safeguarding impact on autism diagnosis linked to vitamin D3 intervention in mothers with early pregnancy 25(OH)D levels of 75nmol/L or higher, as well as the protective connection between elevated gestational serum 25(OH)D levels and reduced autistic symptom load, could not be substantiated through the utilization of the parent-reported Social Responsiveness Scale 2 (SRS-2). SRS-2 scores, impacted by factors unrelated to autism, such as developmental difficulties and behavioral problems, may indicate a general child impairment rather than the severity of core autistic traits.
Thus, compared to clinician ratings, the SRS-2 might be a less specific measure of autistic trait severity, potentially explaining the failure to reproduce the findings. There were no observed connections between maternal post-intervention serum 25(OH)D or child serum 25(OH)D at ages 6 months or 6 years and the risk of autism or ADHD. This contrasts with a recent meta-analysis indicating lower serum 25(OH)D levels in children and adolescents with autism; however, the studies incorporated in that analysis were case-control and susceptible to bias from lifestyle factors.
These factors, such as selective eating habits, reduced outdoor exposure, and medication influencing 25(OH)D levels, may not exert a significant influence on childhood measurements. Another meta-analysis from 2018 suggested an association between low childhood 25(OH)D status and the risk of ADHD, but a Mendelian randomization study did not find evidence supporting a causal relationship.
Elevated maternal preintervention 25(OH)D levels minimize the risk of autism, load of autistic symptoms, and likelihood of ADHD diagnosis. However, administering high-dose vitamin D3 supplementation from the 24th week of pregnancy until 1 week postpartum did not result in an overall reduction in the risk of autism and ADHD diagnosis or symptom load in the offspring at the age of 10, when compared to standard dose vitamin D3.
The American Journal of Clinical Nutrition
High-dose vitamin D3 supplementation in pregnancy and risk of neurodevelopmental disorders in the children at age 10: A randomized clinical trial
Kristina Aagaard et al.
Comments (0)