Categories
Change Password!
Reset Password!


Millions of people around the globe have been affected by COVID-19 infection.
Intradermal injection of the ZyCoV-D vaccine was found to be 66·6% effective against COVID-19.
Millions of people around the globe have been affected by COVID-19 infection. Coronavirus-infected people (majorly <60 years of age) and people who are already suffering from respiratory or cardiovascular conditions are at higher risk of serious complications and death. The rise of the COVID-19 outbreak has elevated the rate of mortality and declined the national economies. Until a large global population is vaccinated, the risk of an upsurge of the SARS-CoV-2 infection and its effects on social and economic life will persist.
Numerous vaccines against coronavirus infection have already been approved but are still not sufficient to control the outburst of SARS-CoV-2 infection. Therefore, the production of vaccines should be done at a large scale, widely utilized in local communities, globally distributed, and affordably priced. Efficient vaccine distribution can be achieved by developing temperature stable vaccines as there would be no requirement for cold storage conditions. ZyCoV-D (a DNA-based vaccine) overcomes the manufacturing and logistics challenges of other RNA-based vaccines.
The key aspect of the ZyCoV-D vaccine is temperature stability. Its storage was done at 2-8°C and was stable at the room temperature (25°C) for at least 3 months, thus retaining all specifications set by Food and Drug Administration and other international guidelines. An open-vial study proved the sterility and stability of ZyCoV-D up to 28 days, which can result in the reduction of the vaccine wastage by eliminating the requirement to wait for multiple people to get the vaccination from a single vial.
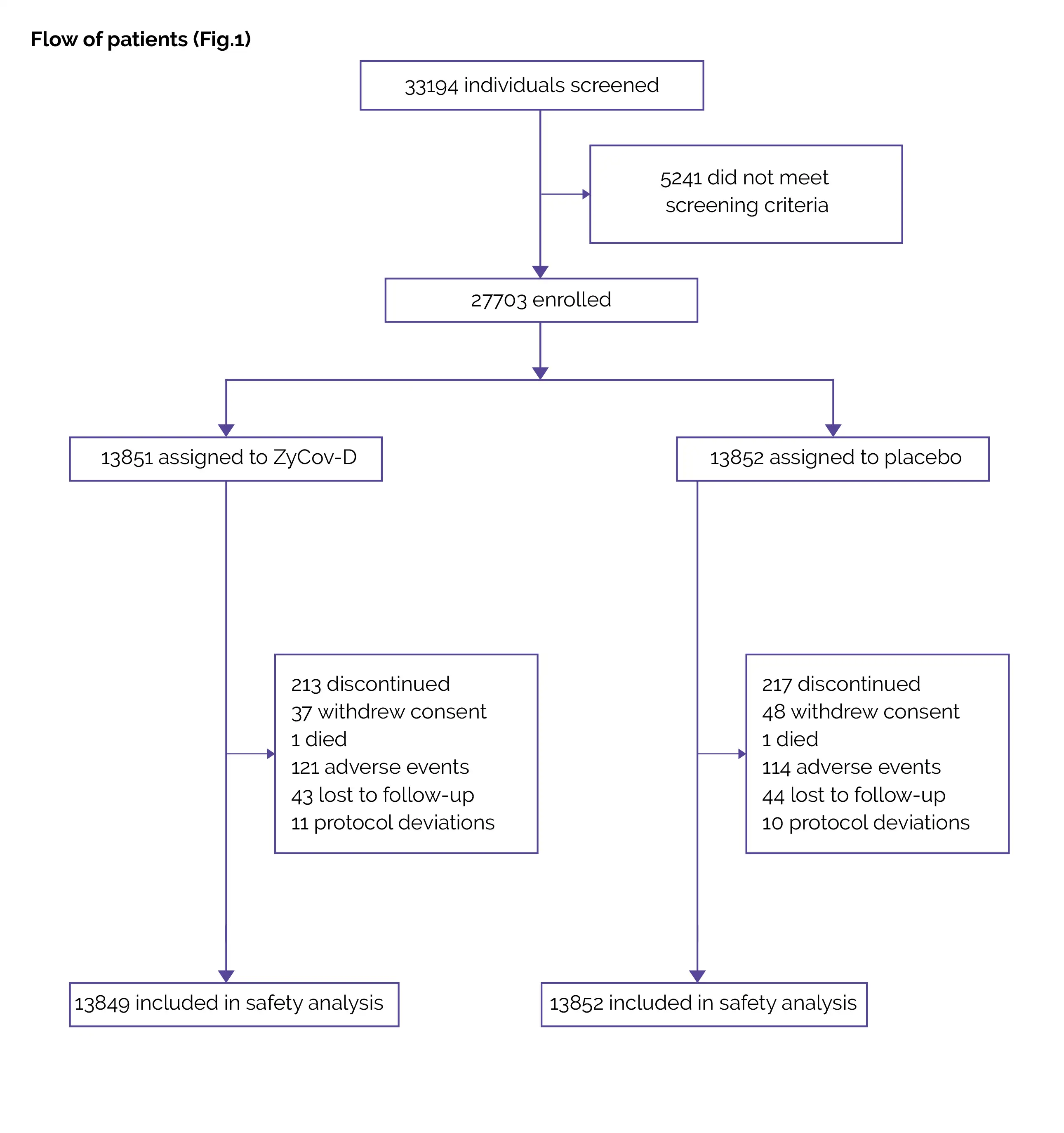
The results of an adaptive phase 1/2 study of ZyCoV-D vaccine in healthy volunteers demonstrated that 43 out of 48 subjects completed the phase 1 study up to day 84, and 911 out of 1000 subjects completed the phase 2 study up to day 224. No safety issues were reported with the administration of the ZyCov-D vaccine up to 2 mg via a needle or needle-free injection system. The vaccine induced a good immune response and strong cellular response. It was discovered to be well tolerated and safe.
RATIONALE BEHIND RESEARCH
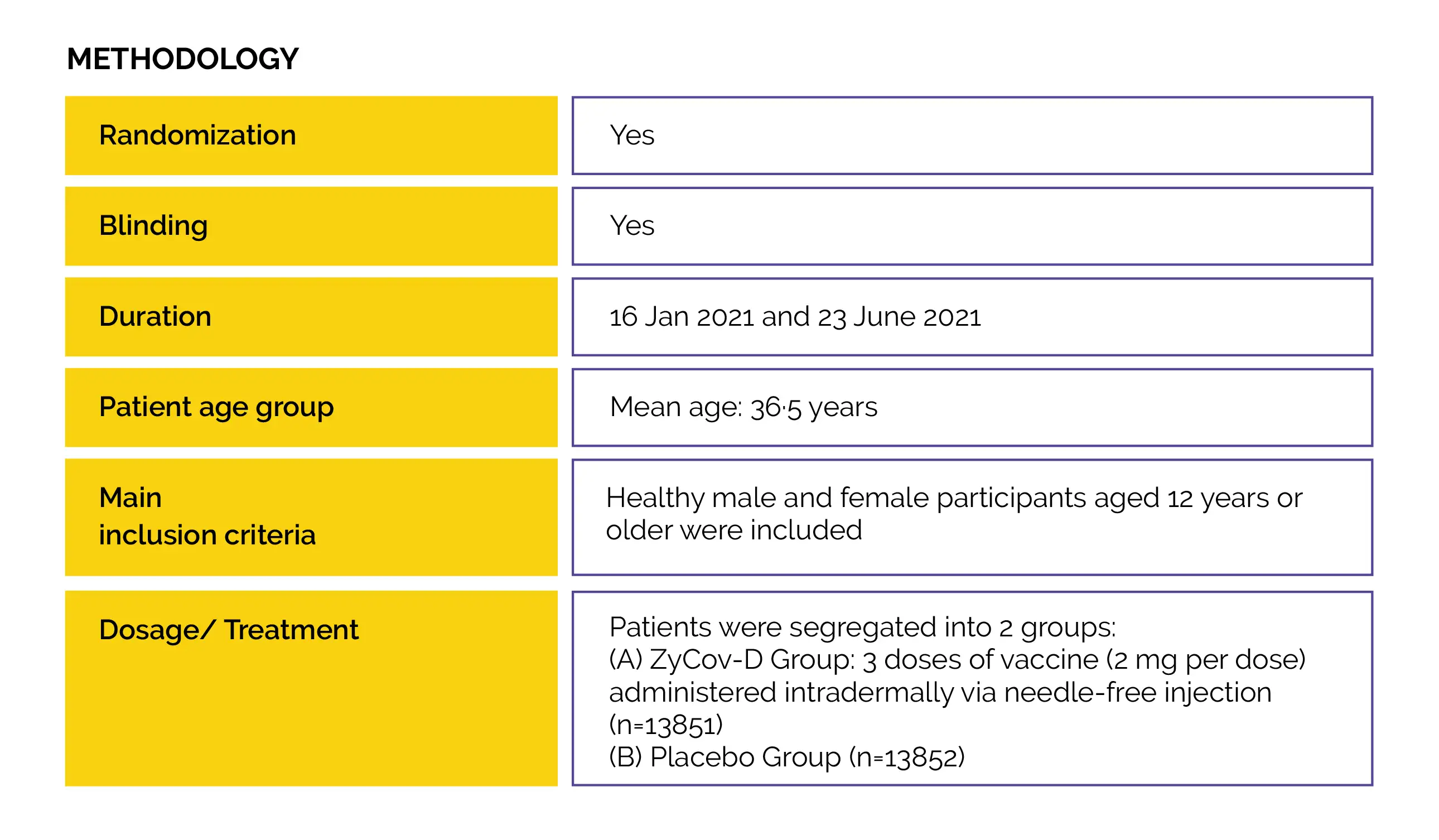
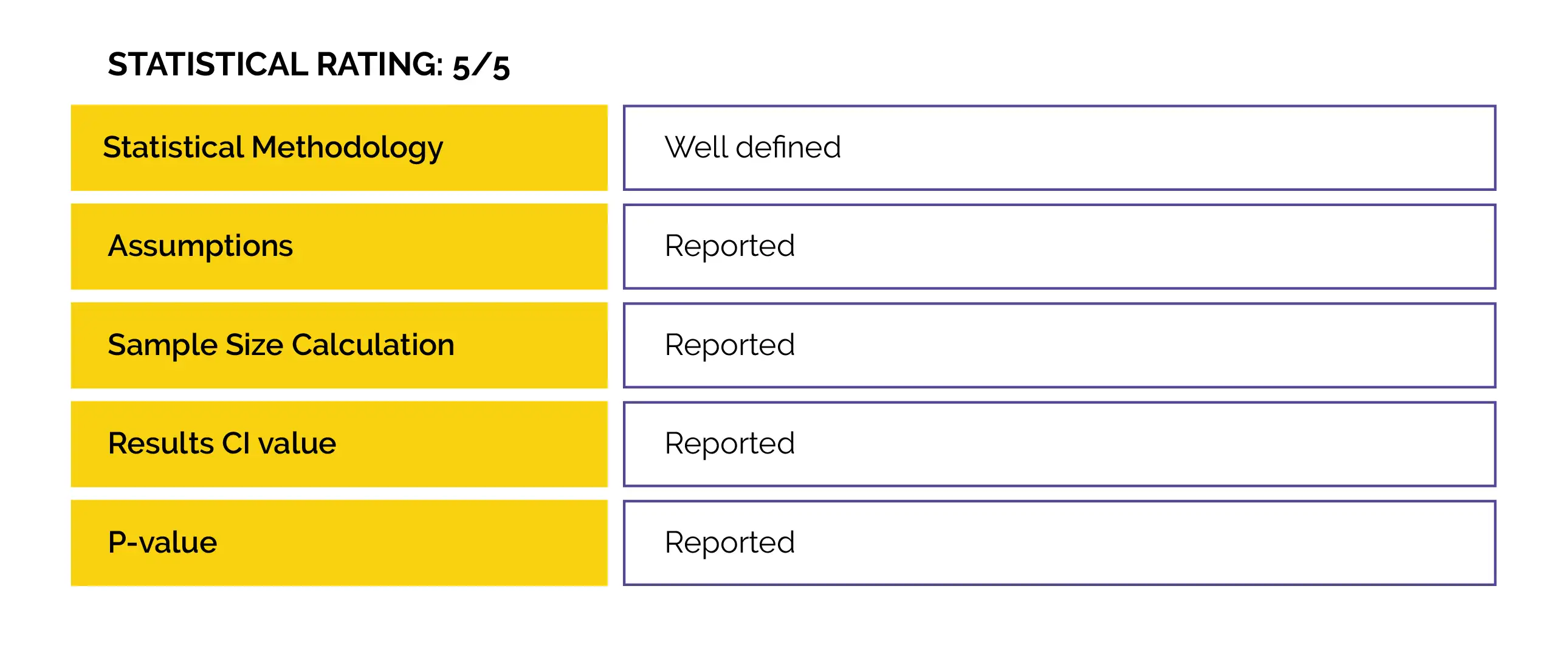
No previous study has investigated the efficacy of a DNA plasmid vaccine on SARS-CoV-2 infection. Thus, this phase 3, randomized, double-blind, placebo-controlled, multicentre study was performed.
OBJECTIVE
An interim analysis was performed to comprehensively assess the efficacy, safety and immunogenicity of the ZyCov-D vaccine against COVID-19.
Study outcomes
Outcomes
Baseline: There were no vital differences reported at baseline.
Study outcomes
Considering the efficacy and safety profile in the phase 1/2 study, the findings from the phase 3 clinical study reported the efficacy, safety, and immunogenicity of the DNA vaccine ZyCoV-D for the prevention of COVID-19. Intradermal administration of the three-dose regimen of the vaccine via a needle-free injection system was 66·6% effective against SARS-CoV-2 infection. The trial was ongoing at the time of the second wave of coronavirus infection which was majorly because of B.1.617.2 (delta) variant.
A rapid elevation in the proportion of delta variant infected people was seen in late April 2021. The ZyCoV-D vaccine is effective against the delta variant. No moderate or severe cases of coronavirus infection were witnessed in the ZyCoV-D group. Hence, the vaccine was considered 100% effective against moderate and severe cases of COVID-19 and 64·9% effective against mild cases of COVID-19. Therefore, with full vaccination utilizing the ZyCoV-D vaccine, prevention of severe and moderate cases of SARS-CoV-2 infection could be done to a considerable extent.
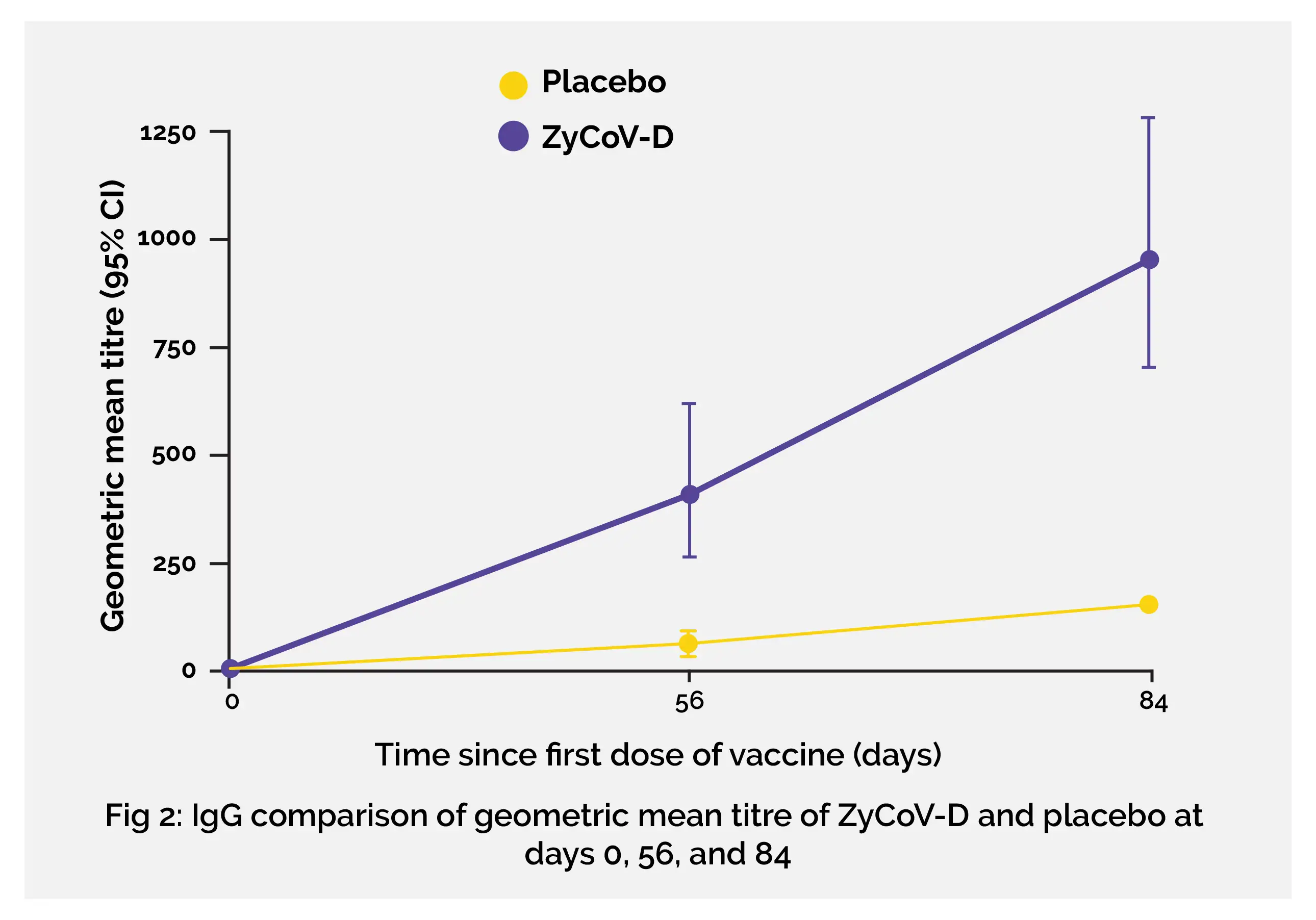
In the phase 3 study, the immunogenicity response of ZyCoV-D vaccine noted in phase 1/2 was maintained. Considerably high immunogenicity response was induced by ZyCoV-D vaccine at day 84 as noticeable from the seroconversion rate (93·33%) on the basis of IgG against S1 antigen (by ELISA) and GMT (952·67 EU, 95% CI 707·9–1282·0) and GMFR (136·09, 95% CI 101·11–183·1) and neutralizing antibody titre (GMT: 133·39 plaque reduction neutralisation test [PRNT50], 95% CI 86·88–204·81; GMFR: 26·68, 95% CI 17·38–40·96). A profound cellular response was induced by the vaccine as evident from elevated levels of interferon-γ in the ZyCoV-D group compared with the placebo group.
The favorable safety profile of ZyCoV-D was confirmed in phase 3 interim analysis as observed in the phase 1/2 trial. The results exhibited that people with comorbidities, people older than 60 years, and people aged 12–17 years showed a comparable tolerability profile of ZyCoV-D with the general study population. No serious toxicity was noted. Major side effects were of mild to moderate intensity and resolved. Itching, swelling, redness, and pain at the injection site were the most common local solicited side effects reported.
Fatigue, muscle pain, fever, and headache were the most common solicited systemic side effects observed. These systemic and local side effects after the administration of the ZyCoV-D vaccine were similar to the placebo group, which indicated that there was no elevated risk of side effects with the vaccine. The severity and type of side effects observed were comparable to those observed in the phase 2 study of another DNA-based vaccine.
Administration of the vaccine via a needle-free injection system should result in reduced adverse events related to needle use, such as pain at the injection site. Storage of ZyCoV-D vaccine is done at 2–8°C, but it is stable at 25°C for a minimum of three months, maintaining all the conditions set by US Food and Drug Administration and other international guidelines. The vaccine's temperature stability aids in the storage and transportation of the vaccine and prevents vaccine wastage by lowering the cold chain breakdown challenges.
Ease of manufacturing with minimal biosafety requirements is offered by the plasmid DNA platform. As ZyCoV-D is a plasmid DNA vaccine, it does not exhibit the issues related to vector-based immunity, for example, poor immune response to target antigens post-vaccination because of pre-existing antibody titres to vectors due to natural infections from vectors like influenza viruses, measles, and adenoviruses.
Other issues related to vector-based immunity include a need for very large doses resulting in numerous adverse events and a requisite for an extended duration between booster doses to reduce the effect of vector immunity interference. The plasmid DNA platform permits the rapid production of new constructs to manage mutations in the virus. The three-dose regimen is equal to a third booster dose.
Intradermal injection of DNA SARS-CoV-2 vaccine (ZyCoV-D) is effective, safe, feasible, immunogenic and may aid to attain successful prevention of COVID-19 disease in a large population. This vaccine has a promising potential to make a significant contribution to curb COVID-19 pandemic.
Lancet
Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India
Akash Khobragade et al.
Comments (0)