Categories
Change Password!
Reset Password!


Lactobacillus plantarum as a probiotic adjuvant can successfully eradicate H. pylori infection.
A recent randomized controlled clinical trial indicated the potential of utilizing probiotics as an adjuvant in eradicating Helicobacter pylori (H. pylori) infection. The research aimed to investigate the efficiency of Lactobacillus plantarum (L. plantarum) as a supplementary treatment in managing H. pylori. This double-blind study included 51 dyspeptic adults (18-70 years old) with confirmed H. pylori infection through gastric biopsies. Participants were treatment-naïve and gave authorization for their involvement, irrespective of gender or ethnicity.
Patients in the probiotic group were subjected to a triple regimen (comprising Amoxicillin 1000 mg administered twice daily for 1 week, Clarithromycin 500 mg twice a day for a week, and Esomeprazole 20 mg taken twice daily for the initial week and subsequently once daily for an additional three weeks) and L. plantarum strain Lp-G18 orally, via capsules with a concentration of 5 x 108 colony forming unit (CFU), once daily for four weeks.
In contrast, the control group was administered a combination of three medications (Amoxicillin 1000 mg twice daily for one week, Clarithromycin 500 mg twice daily for one week, and Esomeprazole 20 mg twice daily for one week followed by once daily for an additional three weeks), along with orally administered placebo capsules containing maltodextrin once daily for a total of four weeks.
The key endpoint ascertained was the elimination of H. pylori, characterized by the histopathological absence of H. pylori bacilli four weeks post-treatment (seven weeks after the completion of triple standard therapy). The secondary endpoint involved assessing the occurrence or absence of adverse events, including alterations in taste/dry mouth, diarrhea, sensations of gas in the abdomen, and epigastric pain/nausea.
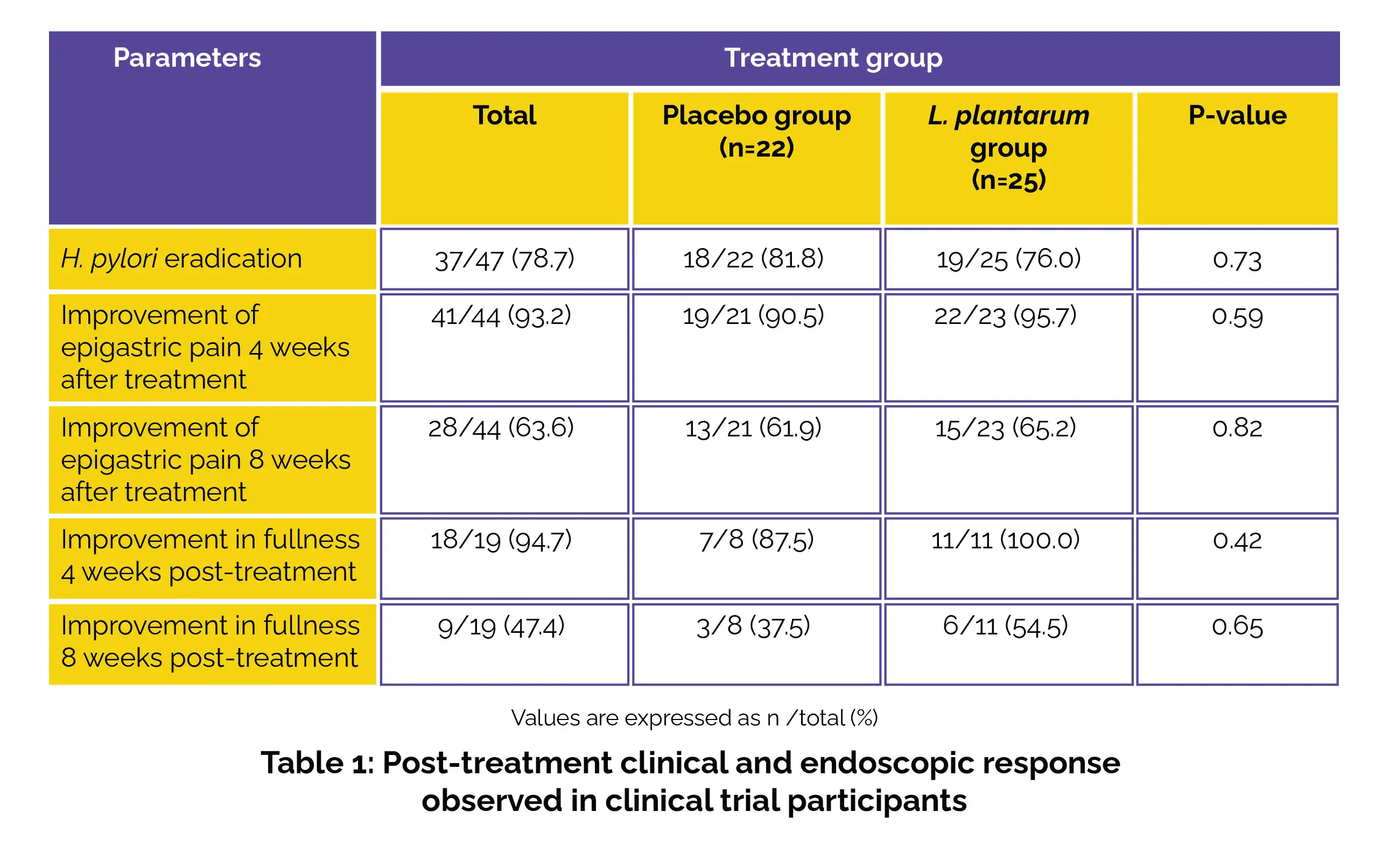
Out of the total participants, this study was completed by 47 subjects. Twenty-seven patients (57.4%) were female. The overall eradication rate stood at 78.7% (n=37), and it exhibited no vital difference between the groups (p = 0.73). Patients reporting initial epigastric pain showed improvement within the first four weeks post-treatment. While more improvements were reported in the L. plantarum group, the difference was not significant. Regarding the enhancement of epigastralgia symptoms in the initial four weeks post-treatment, over half of the participants remained symptom-free after 8 weeks. The endurance of clinical enhancement was also more frequently observed in the cohort that was administered probiotics, albeit without demonstrating a statistically significant distinction.
Concerning the individuals (n=19) who mentioned experiencing gastric fullness during the initial consultation, only one participant in the placebo group did not exhibit improvement in the reported symptoms within the initial four weeks post-treatment. Among those who observed relief from gastric fullness in the initial four weeks following treatment, roughly half remained symptom-free after eight weeks. Although persistent improvement was reported in the probiotic group, statistical confirmation was not obtained (Table 1).
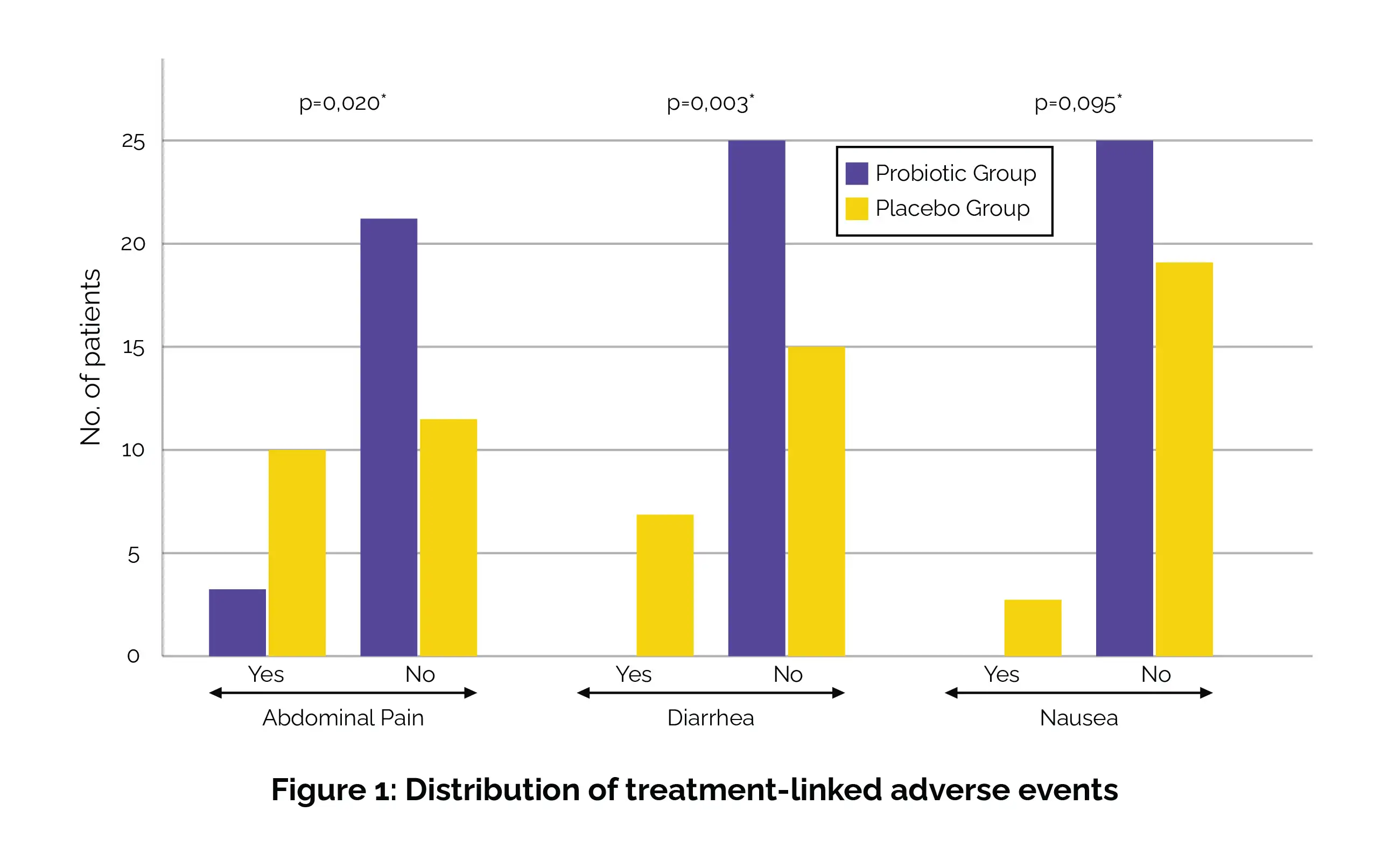
Additional examinations revealed that among individuals up to the age of 45, the elimination rate in those who were administered probiotics was 93.8%, as opposed to 70.0% in the placebo arm (p = 0.046). Regarding the primary adverse events associated with the treatment, it was noted that in the probiotic group, a small proportion of subjects experienced abdominal pain (12%), and none reported diarrhea or nausea. In contrast, within the placebo group, a higher proportion encountered abdominal pain (45.5%), some reported diarrhea (31.8%), and a few developed nausea (13.6%) after initiating the treatment (Figure 1).
This research unveils the possible application of probiotics as a supplement in eradicating H. pylori and prompts exploration into the enhanced collaborative effects of probiotics in younger populations.
Concilium
Lactobacillus plantarum associated with antimicrobial therapy in the eradication of Helicobacter pylori: a randomized clinical trial
Rodrigo Oliveira Ximenes et al.
Comments (0)