Categories
Change Password!
Reset Password!


Continuous upadacitinib therapy was well-tolerated and exhibited a favorable longer-term benefit-risk profile in people having atopic dermatitis.
In an assessment of follow-up data from two ongoing phase 3 trials (Measure Up 1 and Measure Up 2), longer-term upadacitinib therapy showed a promising benefit-risk profile, with sustained effectiveness responses through fifty-two weeks in atopic dermatitis people. This study aimed to assess whether upadacitinib's safety and effectiveness is maintained through fifty-two weeks of continuous therapy in adults and adolescents suffering from moderate to severe atopic dermatitis
Participants were randomly allocated to get once-daily oral upadacitinib 15 mg, 30 mg, or placebo.
At 16th week, subjects randomized at baseline to get 15 mg upadacitinib (273 subjects in Measure Up 1 and 260 subjects in Measure Up 2) and 30 mg (270 subjects in Measure Up 1 and 268 subjects in Measure Up 2) continued the assigned therapy. In a double-blinded manner, the placebo-treated people were rerandomized to get 15 mg upadacitinib (121 subjects in Measure Up 1 and 120 subjects in Measure Up 2) or 30 mg (123 subjects in Measure Up 1 and 121 subjects in Measure Up 2).
Assessment of safety and effectiveness including Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score and 75% improvement in Eczema Area and Severity Index (EASI) was done. Measure Up 1 and Measure Up 2 incorporated 1609 volunteers (727 females; 882 males; mean age 33.8 years). The effectiveness at week 16 was maintained through week fifty-two.
At week fifty-two, the percentage of people who experienced 75% improvement in EASI and who attained vIGA-AD score of clear (0) or almost clear (1) with 2 or higher grades of improvement with the 15-mg dose and 30-mg upadacitinib dose in Measure Up 1 and Measure Up 2, are shown in Table 1.
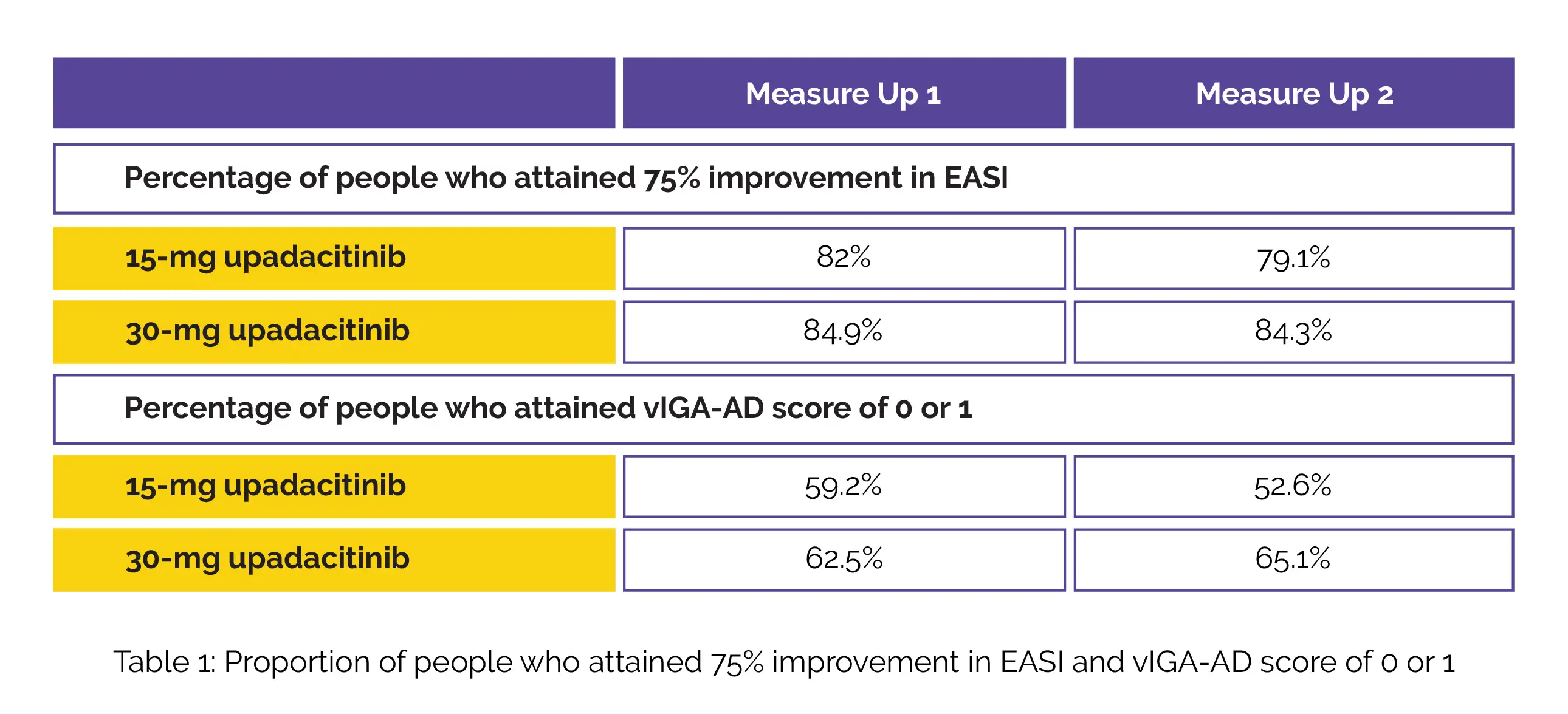
Treatment discontinuation due to adverse events was found to be low overall. However, it was slightly higher for the 30-mg upadacitinib dose. Both the doses of upadacitinib showed good tolerability with no novel safety signals. Thus, the findings through fifty-two weeks illustrated maintenance of efficacy and acceptable longer-term safety of upadacitinib monotherapy for atopic dermatitis management.
JAMA Dermatology
Efficacy and Safety of Upadacitinib in Patients With Moderate to Severe Atopic Dermatitis: Analysis of Follow-up Data From the Measure Up 1 and Measure Up 2 Randomized Clinical Trials
Eric L Simpson et al.
Comments (0)