Categories
Change Password!
Reset Password!


Seladelpar proved to be a promising therapy for patients with primary biliary cholangitis, previously known as primary biliary cirrhosis.
According to a novel clinical trial comprising of patients with primary biliary cholangitis, Seladelpar exhibited considerably superior biochemical response and alkaline phosphatase regularization as compared to the placebo. An improvement in moderate-to-severe itching (pruritus) was also observed with the use of this peroxisome proliferator-activated receptor delta agonist.
A total of 193 patients (with an inappropriate response to or with a history of undesirable side effects with Ursodiol) were randomized (2:1) to receive Seladelpar 10 mg orally every day or placebo.The main endpoint was achieving a biochemical response at one year, while secondary endpoints included normalizing alkaline phosphatase (ALP) levels at one year and evaluating differences in pruritus severity over six months for patients with moderate-to-severe itchiness at the beginning of the trial.
Of the total patients, 93.8% received Ursodiol as standard background therapy. Compared to the placebo group, more patients in the Seladelpar group encountered a biochemical response. Normalization of ALP levels was also more common in the Seladelpar group (P<0.001) as shown in Table 1:
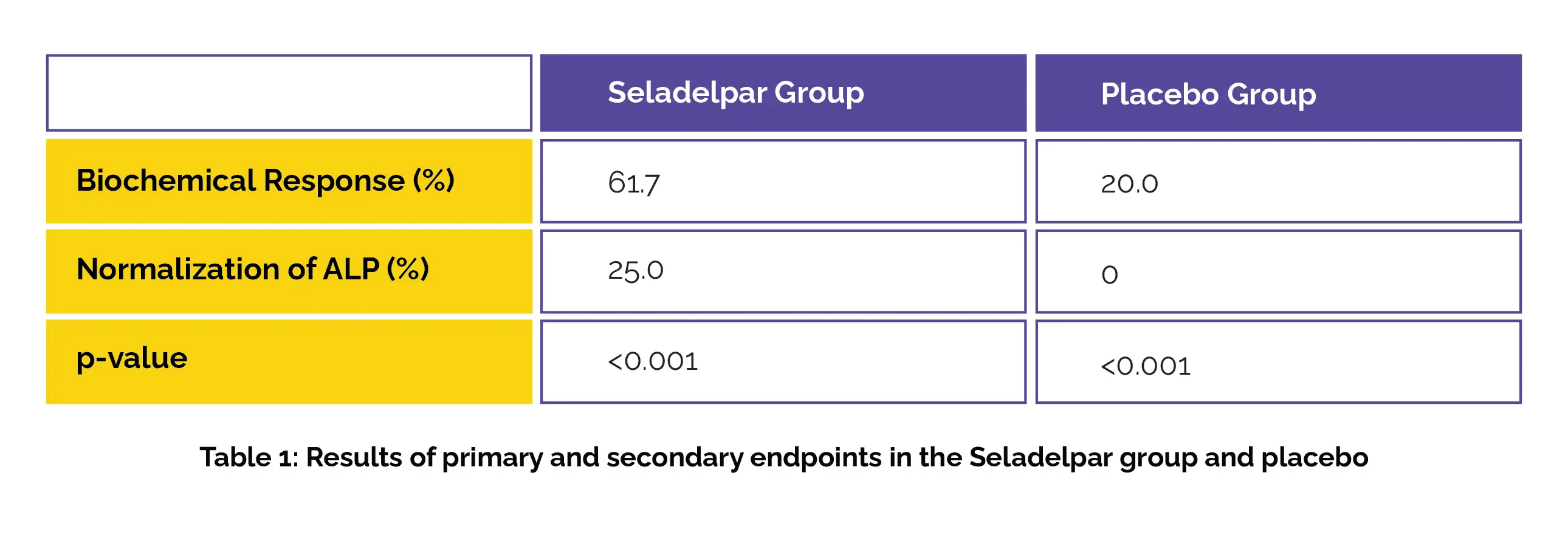
Furthermore, Seladelpar resulted in a larger decrease in pruritus severity than placebo. The adverse events were described in 86.7% and 84.6% of patients in the Seladelpar and placebo groups, with severe events occurring in 7% and 6.2% correspondingly.
The New England Journal of Medicine
A Phase 3 Trial of Seladelpar in Primary Biliary Cholangitis
Gideon M Hirschfield et al.
Comments (0)