Categories
Change Password!
Reset Password!


Treatment with CD24Fc led to sustained improvements in time to clinical improvement, decreased disease progression and shortened length of hospital stay in COVID-19 people receiving oxygen support.
In comparison with placebo, a single intravenous infusion of CD24Fc was well-tolerated and exhibited remarkable efficacy in accelerating clinical improvement and decreasing disease advancement in COVID-19 people, as elucidated from a placebo-controlled, phase 3, randomized, double-blind study. Researchers undertook this study to determine the safety and effectiveness of the immunomodulator CD24Fc (480 mg) in hospitalized adults (age ≥18 years) with SARS-CoV-2 infection and receiving oxygen support and standard of care.
COVID-19 people who were at risk of advancement to severe and critical illness were randomly allocated by site-stratified block randomization to get CD24Fc or placebo. The time to clinical improvement over twenty-eight days was the major outcome ascertained. The prespecified primary interim assessment was carried out when 146 subjects reached the time to clinical improvement outcome. In the intention-to-treat population, assessment of efficacy was done while in the as-treated population, evaluation of safety was carried out.
Overall, 243 hospitalized people were evaluated for eligibility and 234 were recruited and randomized to get placebo (n=118) or CD24Fc (n=116). In the interim assessment, the 28-day clinical improvement rate and the median time to clinical improvement in CD24Fc and placebo arms, are shown in Table 1:
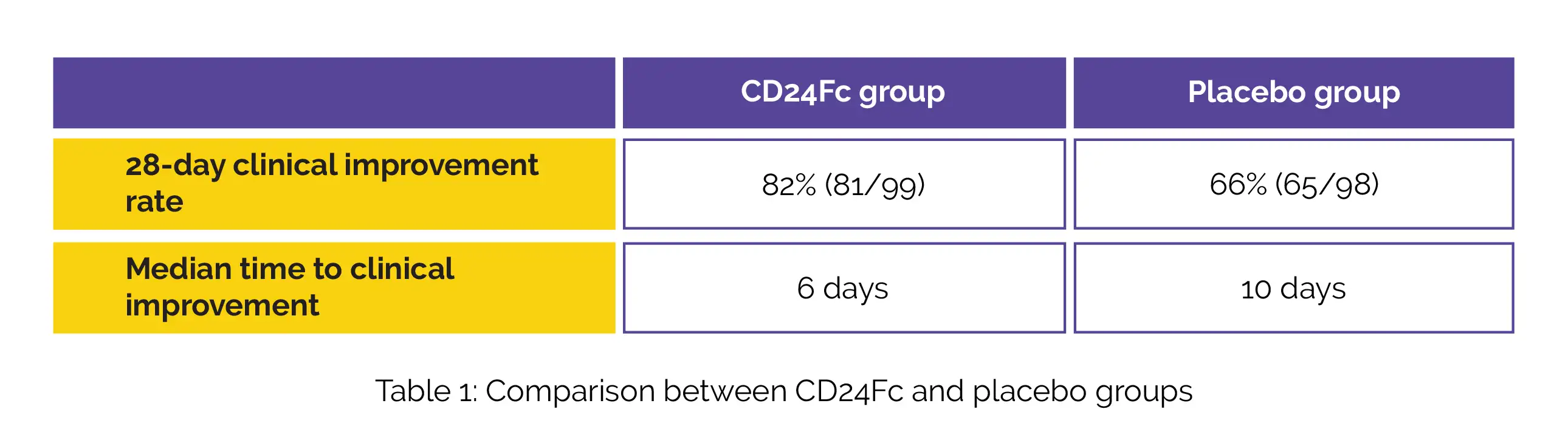
Overall, 37 subjects were randomized after the interim assessment data cutoff date. In the 234 randomized subjects, the median time to clinical improvement was 6·0 days in CD24Fc arm when compared to 10·5 days in placebo arm. The percentage of people with disease advancement within 28 days was 31% (36/118) in placebo arm and 19% (22/116) in CD24Fc arm.
In both CD24Fc and placebo arms, the incidence of adverse events and severe adverse events were comparable. There were no treatment-linked adverse reactions. The findings signify that targeting tissue injuries-elicited inflammation may offer a promising choice for people hospitalized due to coronavirus disease.
The Lancet Infectious Diseases
Efficacy and safety of CD24Fc in hospitalised patients with COVID-19: a randomised, double-blind, placebo-controlled, phase 3 study
James Welker et al.
Comments (0)