Categories
Change Password!
Reset Password!


Crisaborole improves atopic dermatitis symptoms in Asian patients aged ≥2 years with atopic dermatitis.
Crisaborole 2% ointment is recommended for its potential role in treating atopic dermatitis (AD) or eczema of mild to moderate severity.
This post-analysis of CrisADe CLEAR study published in ‘Dermatology and Therapy’ journal was performed to test the efficacy and safety of Crisaborole in 391 patients aged more than or equal to 2 years. Of these, the patients either received Crisaborole or a placebo to be used two times everyday for 28 days. The main endpoint of the study was the percentage change from baseline in Eczema areas and Severity Index (EASI) score on the 29th day. The secondary endpoint was to examine the difference in the weekly average Peak Pruritus Numerical Rating Scale (PP-NRS) score along with the improvement in the Investigator’s Static Global Assessment (ISGA) score.
The results favoured the use of Crisaborole as a greater drop in percentage change from baseline in EASI score and; elevated response rates for attainment of ISGA improvement and ISGA success were observed in those who applied the Crisabrole ointment. A greater reduction in weekly average PP-NRS score was also reported (details in Table 1) with the use of this nonsteroidal phosphodiesterase 4 inhibitor.
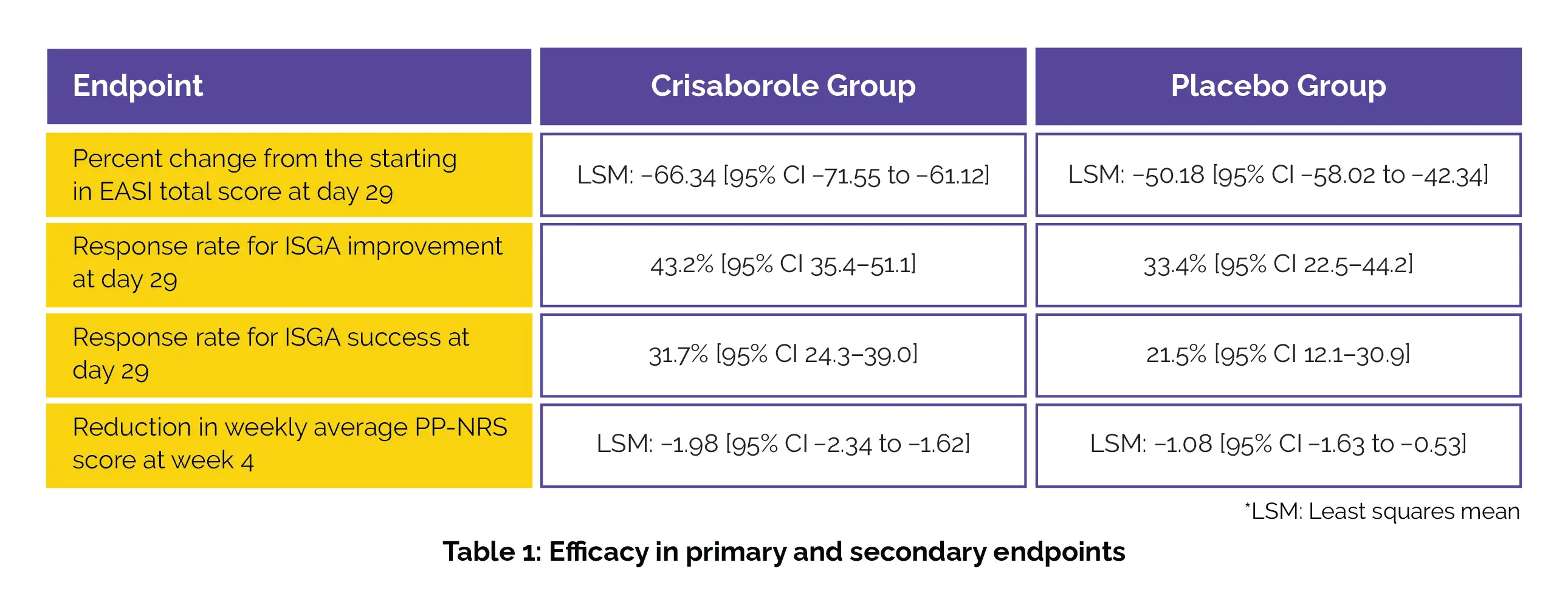
Thus proving the effectiveness of Crisaborole in >2-year-old or above patients with AD, as mentioned.
Dermatology and Therapy
Efficacy and Safety of Crisaborole Ointment 2% in Chinese Patients Aged ≥ 2 Years with Mild to Moderate Atopic Dermatitis
Lin Ma et.al
Comments (0)