Categories
Change Password!
Reset Password!


Benralizumab shows promise in achieving histological remission for eosinophilic gastritis, paving the way for innovative treatment approaches.
In a revolutionary study published in “The Lancet Gastroenterology & Hepatology”, researchers have unveiled a potential game-changer in the treatment of eosinophilic gastritis. The study, led by Kara L Kliewer, sheds light on the elusive role of eosinophils in gastrointestinal diseases and presents a new approach that could modernize patient outcomes. The administration of Benralizumab resulted in a notable achievement of histological remission, characterized by the absence of tissue eosinophilia, among a majority of patients suffering from eosinophilic gastritis.
This placebo-controlled, randomized, double-blind study examined the effectiveness and safety of Benralizumab in individuals diagnosed with eosinophilic gastritis. People in the age group of 12 to 60 years having symptomatic, histologically active eosinophilic gastritis and blood eosinophilia were included. They were erratically assigned to receive either Benralizumab dosed at 30 mg or placebo subcutaneously once every 4 weeks for a total of 12 weeks (3 injections). The main focus of the study was to determine the percentage of patients who achieved histological remission (defined as a peak gastric eosinophil count of less than 30 eosinophils per high-power field) by the 12th week.
Additionally, the study's secondary objectives included examining alterations in peak eosinophil count, histologic eosinophilic gastritis, the Eosinophilic Gastritis Endoscopic Reference System (EG-REFS) score, and patient-reported symptom assessments (such as the Severity of Dyspepsia Assessment [SODA] and the collection of patient-centered tools known as the Patient-Reported Outcome Measurement Information System [PROMIS]) from baseline to week 12.
Patients met the criteria for enrollment in two open-label extension (OLE) phases, extending until week 88. During these phases, all patients were administered Benralizumab subsequent to the initial 12-week double-blind period. Thirty-four patients were screened. Twenty-six patients were randomly assigned to either Benralizumab or placebo group (13 patients in each group). The details of histological remission have been portrayed in Table 1:
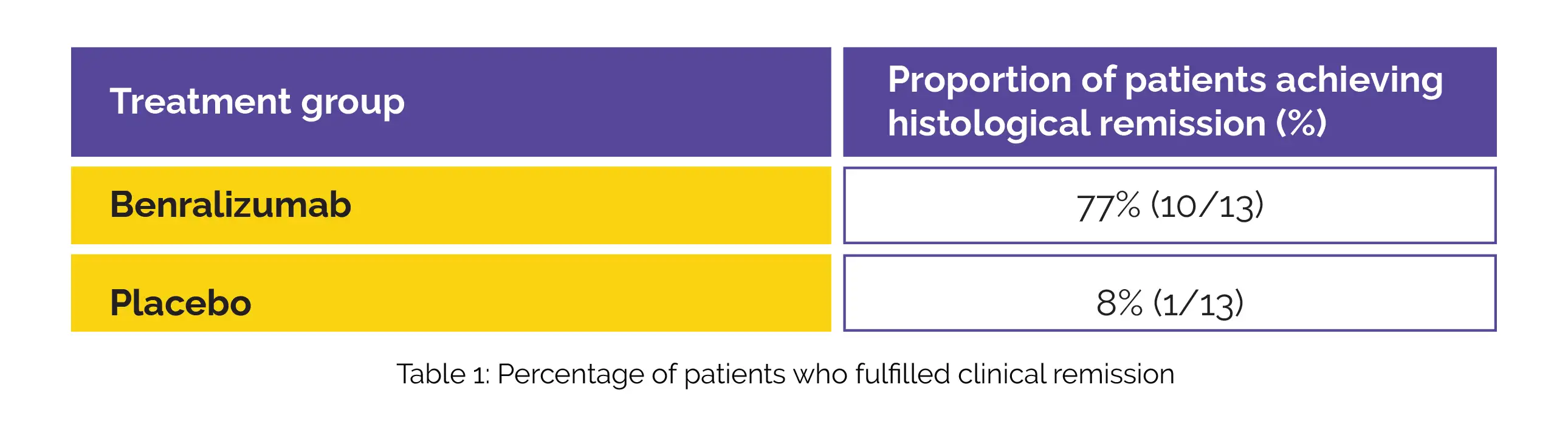
Notably, the Benralizumab group exhibited considerably larger changes from baseline to week 12 than the placebo group across various parameters under consideration such as peak gastric eosinophil counts, eosinophilic gastritis histology total score, histology inflammatory score, and blood eosinophil counts. The eosinophilic gastritis histology structural score, EG-REFS score, or patient-reported outcomes changes were not significant. The instances of treatment-emergent adverse events were prevalent in 11 out of 13 patients (85%) within the Benralizumab group, while six out of 13 patients (46%) within the placebo group experienced similar events.
Headache, nausea, and vomiting were the most frequently observed adverse events. No treatment-related deaths were noted. Neither of the severe adverse events (dizziness and rhabdomyolysis; aspiration) were related to the treatment. While the road ahead may still hold challenges, the emergence of Benralizumab as a potential game-changer in eosinophilic gastritis treatment cannot be ignored. This study marks a significant leap forward in our understanding of the disease and offers new hope for patients longing for relief from the burden of eosinophilic gastritis.
Despite this positive development, the continued presence of certain histological, endoscopic, and other disease-related attributes hints at the existence of an additional, eosinophil-independent pathway contributing to the disease's progression. These findings underscore the necessity for a more comprehensive approach that encompasses a broader targeting of type 2 immunity to address the multifaceted nature of this condition.
The Lancet Gastroenterology & Hepatology
Benralizumab for eosinophilic gastritis: a single-site, randomised, double-blind, placebo-controlled, phase 2 trial
Kara L Kliewer et al.
Comments (0)