Categories
Change Password!
Reset Password!


To assess the safety and efficacy of Tapinarof 1% cream in adult patients with plaque psoriasis through two phase 3 studies, ZBA4-1 and ZBA4-2.
Topical application of Tapinarof effectively manages plaque psoriasis over 52 weeks, showing sustained efficacy and a favorable safety profile for long-term use.
To assess the safety and efficacy of Tapinarof 1% cream in adult patients with plaque psoriasis through two phase 3 studies, ZBA4-1 and ZBA4-2.
In the ZBA4-1 study (number of patients= 158), participants underwent a 12-week, double-blind, vehicle-controlled treatment phase (course 1) where they were randomly assigned in a 2:1 ratio to receive either Tapinarof or a vehicle. It was followed by a 12-week extension phase (course 2) where all participants received Tapinarof. The ZBA4-2 study (number of patients= 305) was a 52-week, open-label, uncontrolled trial where all participants were treated with Tapinarof.
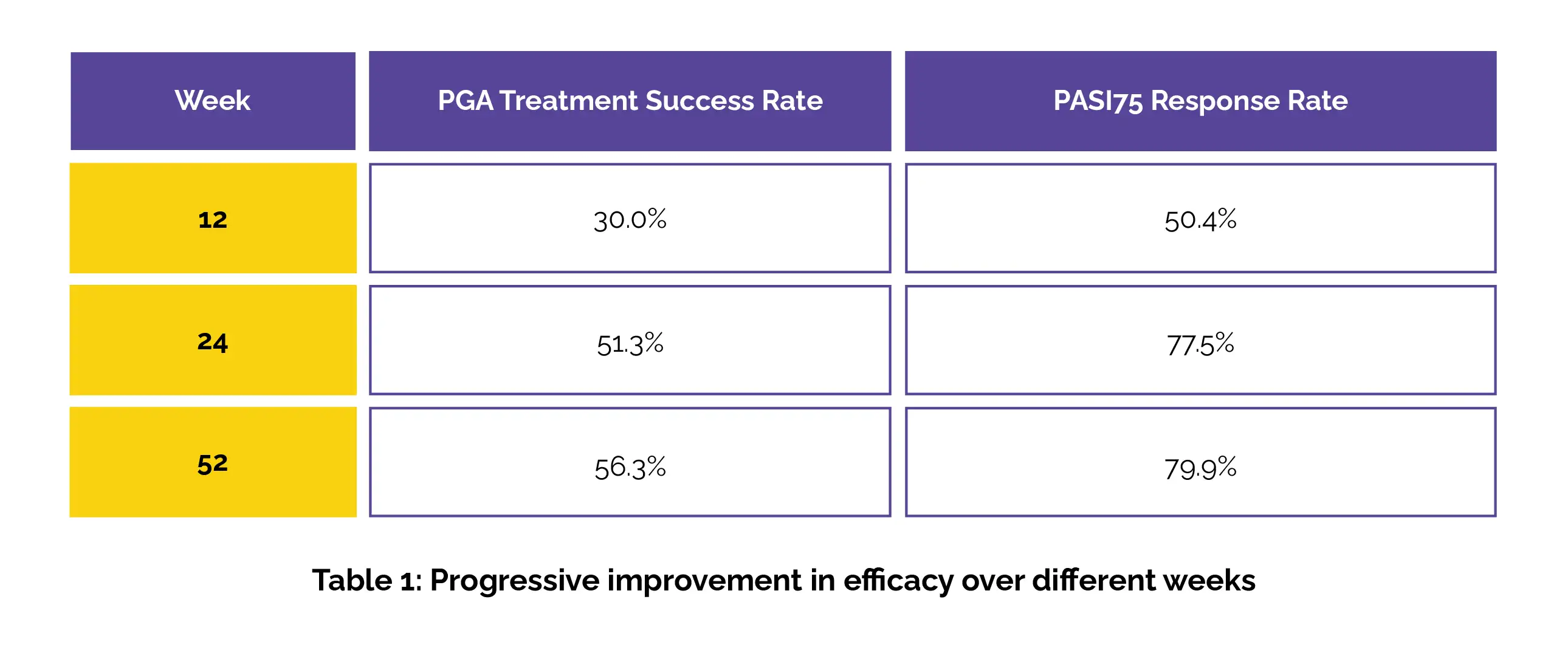
In the ZBA4-1 study, in the first course, a PGA i.e. 6-point Physician's Global Assessment score of 0 (clear) or 1 (almost clear) with a ≥2-grade improvement from the baseline at week 12 was achieved by 20.06% of patients in the Tapinarof group, compared to only 2.50% in the other group. Furthermore, 37.7% of patients in the Tapinarof group reached a Psoriasis Area and Severity Index (PASI) 75 response, while only 3.8% in the other group did so at week 12. In the ZBA4-2 study, the PGA treatment success rate and PASI75 response rate at weeks 12, 24, and 52 have been portrayed in the following Table 1:
Across both studies, most adverse events were mild or moderate, with folliculitis and contact dermatitis being frequently encountered.
Tapinarof (non-steroidal, topical, aryl hydrocarbon receptor agonist) cream was effective and generally safe for about 12 months in plaque psoriasis-affected patients.
The Journal of Dermatology
Tapinarof cream for the treatment of plaque psoriasis: Efficacy and safety results from 2 Japanese phase 3 trials
Atsuyuki Igarashi et al.
Comments (0)