Categories
Change Password!
Reset Password!


Cannabidiol (CBD), a plant-derived compound, has been making headlines for its potential in treating epilepsy. The CBD Expanded Access Program offered compassionate access to CBD for individuals suffering from treatment-resistant epilepsy, including tuberous sclerosis complex (TSC). This study dived into the effectiveness and safety of CBD over an extended period for patients with TSC, a condition often resistant to conventional treatments.
Cannabidiol shows promising long-term benefits in reducing seizures for patients with tuberous sclerosis complex, with manageable side effects.
Cannabidiol (CBD), a plant-derived compound, has been making headlines for its potential in treating epilepsy. The CBD Expanded Access Program offered compassionate access to CBD for individuals suffering from treatment-resistant epilepsy, including tuberous sclerosis complex (TSC). This study dived into the effectiveness and safety of CBD over an extended period for patients with TSC, a condition often resistant to conventional treatments.
In the study, patients received CBD oil as an add-on treatment. Starting doses ranged from 2 to 10 mg/kg/day, with adjustments up to 25-50 mg/kg/day based on tolerance. Efficacy was measured by alteration in seizure frequency and responder rates over 12-week intervals up to 144 weeks. Additionally, the safety was tracked through 233 weeks.
Thirty-four TSC patients, aged 1.8 to 31.2 years, participated in the study. They were on a median of three antiseizure medications before starting CBD. The median CBD dose ranged from 25-28 mg/kg/day initially to 20-50 mg/kg/day later on. Seizure reduction was significant in all types of seizures as shown in Table 1 below:
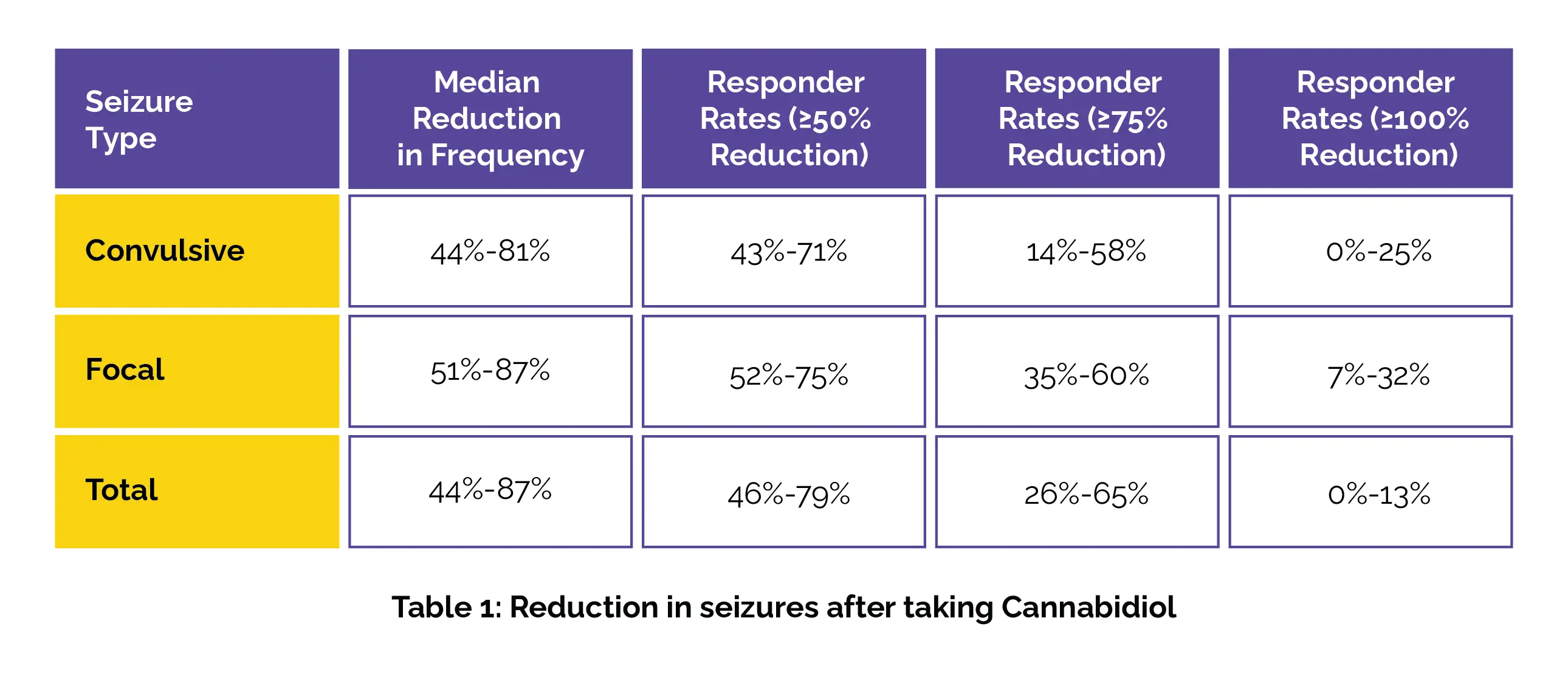
Adverse events (AEs) were common, with 94% of patients experiencing at least one. Serious AEs, deemed unrelated to treatment, affected 47%, while 71% had treatment-related AEs, including sleepiness, diarrhea, and ataxia. Two patients discontinued due to AEs, but there were no deaths.
Long-term use of CBD was effective in reducing seizure frequency in TSC patients, with a safety profile consistent with previous studies. The results highlighted CBD’s potential as a valuable addition to epilepsy treatment regimens, offering significant benefits with generally mild to moderate side effects.
Epilepsia open
Long-term efficacy and safety of cannabidiol in patients with tuberous sclerosis complex: 3-year results from the cannabidiol expanded access program
Arie Weinstock et al.
Comments (0)