Categories
Change Password!
Reset Password!


A recent phase III study showed that tanezumab therapy and nonsteroidal anti-inflammatory drugs (NSAIDs) improve pain and physical function in hip or knee osteoarthritis. In this study, time course and clinical significance of these initial findings were assessed utilizing a mixture of primary, secondary, and post hoc outcomes.
Treatment with subcutaneous tanezumab or oral NSAIDs offers early and sustained effectiveness relative to baseline in people with moderate-to-severe osteoarthritis of hip or knee and a past history of unsatisfactory response to standard osteoarthritis analgesics.
A recent phase III study showed that tanezumab therapy and nonsteroidal anti-inflammatory drugs (NSAIDs) improve pain and physical function in hip or knee osteoarthritis. In this study, time course and clinical significance of these initial findings were assessed utilizing a mixture of primary, secondary, and post hoc outcomes.
People on a stable dose of NSAIDs and with a past history of unsatisfactory response to other standard osteoarthritis analgesics were recruited in an 80-week (fifty-six weeks treatment/twenty-four weeks safety follow-up), randomized, NSAID-controlled, phase III study. This study investigated tanezumab's long-term safety for hip or knee osteoarthritis. The volunteers received NSAIDs (twice daily diclofenac, naproxen, or celecoxib) or tanezumab (2.5 mg or 5 mg every eight weeks).
The non-responders were discontinued at the 16th week. The alteration from the baseline in Patient's Global Assessment of Osteoarthritis (PGA-OA), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain and Physical Function, and average pain in index joint were comparatively evaluated between tanezumab and NSAID groups over the fifty-six week therapeutic period. Safety, rescue medication usage, and statistically significant responses (such as ≥30% and ≥50% betterment in WOMAC pain and physical function) were also examined.
Over the 56-week therapeutic period, all the groups showed improvement in PGA-OA, WOMAC pain (Figure 1), WOMAC physical function, and average pain in the index joint when compared to the baseline.
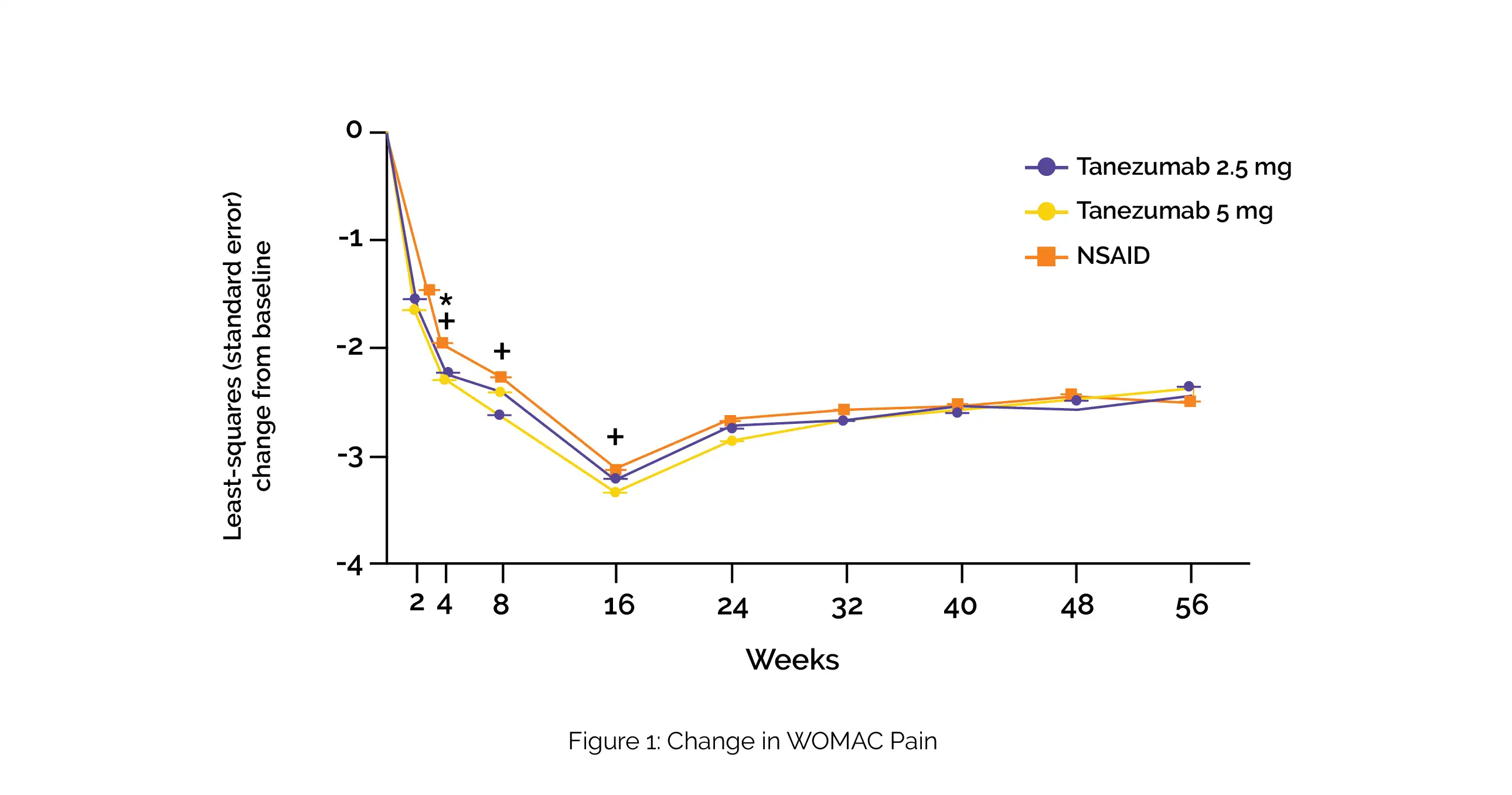
Across all the study groups, the improvements usually occurred from the time of the 1st evaluation (first or second) to 16th week and then marginally reduced from week 16 to 24 prior to stabilizing from weeks 24 to 56.
The extent of improvement and the percentage of people attaining ≥30% and ≥50% enhancement in these outcomes was higher with tanezumab therapy when compared to NSAIDs at some timepoints on or prior to the 16th week. Adverse effects of abnormal peripheral sensation, prespecified joint safety events, and total joint replacement surgery were found to occur more commonly with tanezumab vs NSAIDs.
Tanezumab (a nerve growth factor inhibitor) and NSAIDs both resulted in early and sustained effectiveness relative to the baseline. In a substantial percentage of volunteers, there were remarkable improvements in pain and function.
Arthritis Research & Therapy
Observed efficacy and clinically important improvements in participants with osteoarthritis treated with subcutaneous tanezumab: results from a 56-week randomized NSAID-controlled study
Tuhina Neogi et al.
Comments (0)