Categories
Change Password!
Reset Password!


A randomized phase 3b trial was conducted to investigate the magnitude and durability of clinical effects of the triple combination regimen in patients (age 12 years and above) suffering from cystic fibrosis and who were homozygous for the F508del-CFTR mutation.
In patients with cystic fibrosis, the triple combination regimen (elexacaftor + tezacaftor + ivacaftor) is safe, well-tolerated and has superior efficacy when compared to tezacaftor + ivacaftor regimen.
A randomized phase 3b trial was conducted to investigate the magnitude and durability of clinical effects of the triple combination regimen in patients (age 12 years and above) suffering from cystic fibrosis and who were homozygous for the F508del-CFTR mutation.
This double-blind, active-controlled, multicentre trial recruited 176 participants with stable disease and forced expiratory volume (FEV1) of 40–90%. Following a 4-week run-in period in which the subjects were given 100 mg tezacaftor orally once daily (OD) and 150 mg ivacaftor every 12 hours, 175 subjects were randomly allocated in the ratio of 1:1 to get 24 weeks of either 200 mg elexacaftor orally OD + 100 mg tezacaftor orally OD + 150 mg ivacaftor orally every 12 hours (elexacaftor + tezacaftor + ivacaftor, Group 1, n=87) or 100 mg tezacaftor orally OD + 150 mg ivacaftor orally every 12 hours (tezacaftor + ivacaftor, Group 2, n=88).
During the screening visit, randomization was graded by percent predicted FEV1, age, and if the subject was taking cystic fibrosis transmembrane conductance regulator (CFTR) modulators.
The absolute alteration in Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score from baseline till week 24 was the major outcome ascertained while the absolute alteration in percent predicted FEV1 from baseline till week 24 was the secondary outcome ascertained. Tolerability, safety, and the absolute alteration from baseline in sweat chloride concentrations till week 24 were the other secondary outcomes.
From the baseline till week 24, an increase in mean CFQ-R score and mean percent predicted FEV1 was noted in both groups. On the other hand, a decrease in mean sweat chloride concentration was reported in both groups. Most subjects reported mild-to-moderate side effects. Severe side effects were also reported, as shown in Table 1:
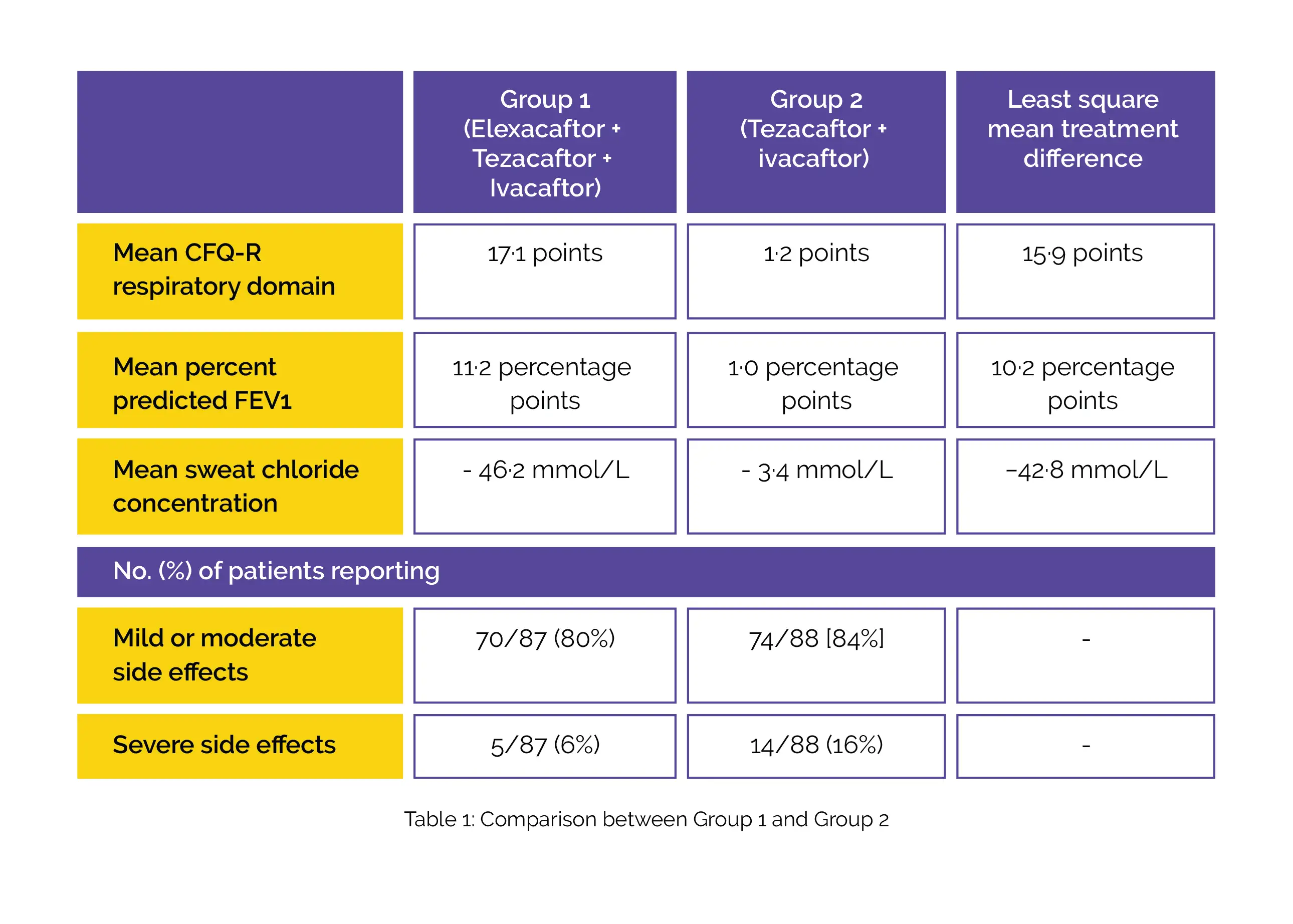
Notably, 1 subject in Group 1 terminated the treatment because of a side effect of depression and anxiety and 2 subjects in Group 2 discontinued therapy (1 patient suffered from psychotic disorder and 1 patient suffered from obsessive-compulsive disorder).
Hence, the triple combination regimen showed good tolerability and safety in people having cystic fibrosis. Furthermore, it incited improvements in CFTR functions, respiratory-linked quality of life, and lung function. The changes were durable over 24 weeks and showed superiority to the tezacaftor+ ivacaftor regimen.
The Lancet Respiratory Medicine
Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: a 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial
Sivagurunathan Sutharsan et al.
Comments (0)