Categories
Change Password!
Reset Password!


To study the efficacy and safety of once-daily administration of Roflumilast, a selective, highly potent phosphodiesterase 4 inhibitor for atopic dermatitis (AD) in children and adults.
Roflumilast 0.15% topical cream offers a promising option for patients seeking non-systemic therapies, showing significant itch relief, and improving sleep and overall quality of life.
To study the efficacy and safety of once-daily administration of Roflumilast, a selective, highly potent phosphodiesterase 4 inhibitor for atopic dermatitis (AD) in children and adults.
The combined data from two identical Phase 3 randomized controlled trials (INTEGUMENT-1 and -2) evaluating Roflumilast 0.15% cream were considered. The patients aged 6 years and older with mild to moderate AD were randomly allocated in a 2:1 ratio to receive either Roflumilast (number of patients=884) or a placebo cream (number of patients=453) to be used once daily for a total of 4 weeks.
The primary outcome was the validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) success from the start at week 4. Tools such as the self-assessed Patient Oriented Eczema Measure, Worst Itch-Numeric Rating Scale (WI-NRS) questionnaire, SCORing AD (SCORAD), 10-question Dermatology Life Quality Index (DLQI)/Children’s DLQI and Dermatitis Family Impact questionnaire were used to assess the patient-reported measures. An examination of the safety and tolerability was also done.
Compared to those on the placebo cream, a higher percentage of patients treated with Roflumilast achieved vIGA-AD success, WI-NRS success, and WI-NRS of 0/1 at week 4 (shown in Table 1):
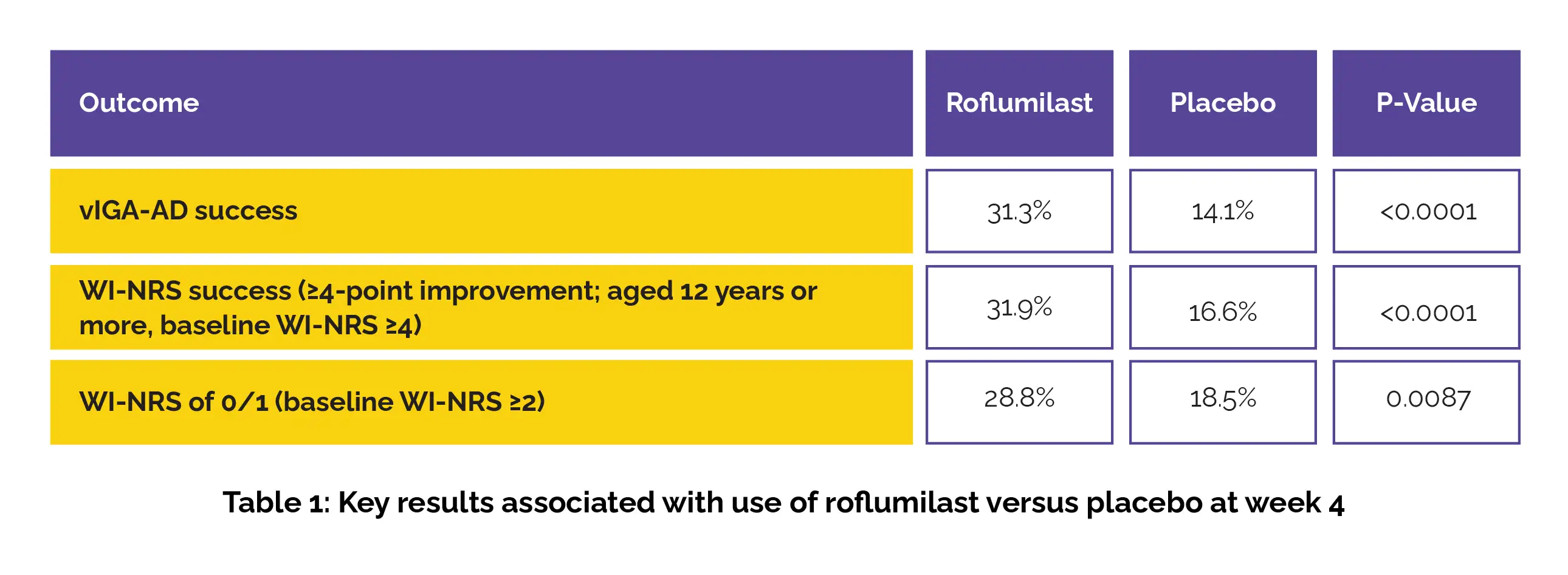
As per the SCORAD, the Roflumilast-treated patients also showed greater improvements in pruritus and sleep scores compared to the vehicle group (least squares mean percentage change of −48.2% vs. −27.8%, P<0.0001, and −47.9% vs. −22.9%, P<0.05, respectively). Additionally, Roflumilast demonstrated positive effects on other secondary endpoints, including the quality of life of the patient and the family. Favorable adverse effects profile and local tolerability were reported.
Nonsteroidal Roflumilast cream 0.15% when used once every day leads to improvements in patient-reported measures in individuals with itchy, inflamed skin.
British Journal of Dermatology
Pooled efficacy, patient-reported outcomes, and safety of roflumilast cream 0.15% from the INTEGUMENT-1 and INTEGUMENT-2 phase 3 trials of adults and children with atopic dermatitis
Eric Simpson et al.
Comments (0)