Categories
Change Password!
Reset Password!


A multicenter, retrospective, 1-year real-life study aimed to examine the safety and efficacy of risankizumab in moderate-to-severe psoriasis patients.
In people suffering from psoriasis, risankizumab exhibited a favorable safety profile and high efficacy, irrespective of disease- and patient-related factors.
A multicenter, retrospective, 1-year real-life study aimed to examine the safety and efficacy of risankizumab in moderate-to-severe psoriasis patients.
This study was carried out on 112 people with chronic plaque psoriasis who were given risankizumab therapy. At baseline and after 4, 16, 28 and 52 weeks, measurement of Psoriasis Area and Severity Index (PASI) was done.
Assessment of clinical responses by PASI75, PASI90 and PASI100 was done at the same time points. Collection of side effects and possible safety issues was done. For variables predicting clinical response, multivariable and univariable logistic regressions were carried out.
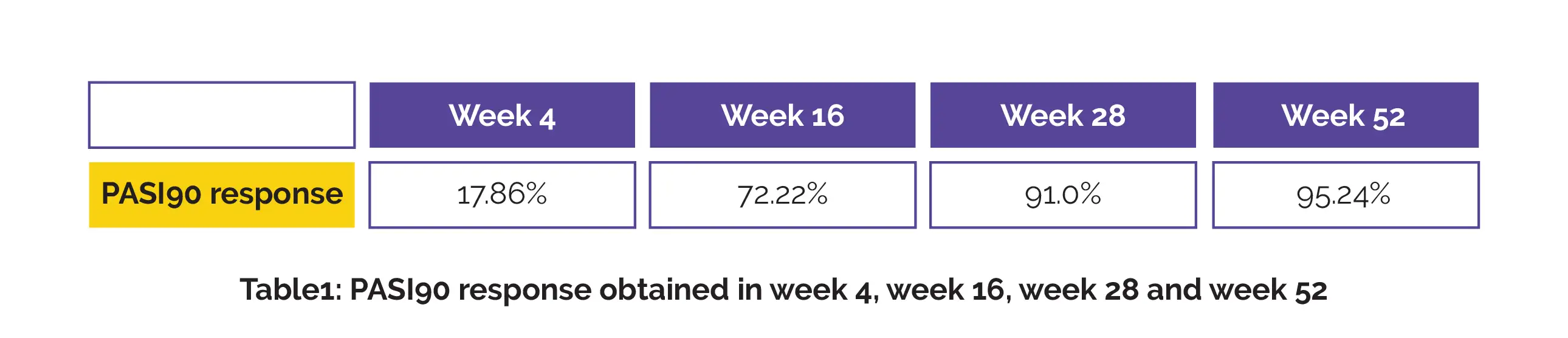
The PASI90 response obtained in week 4, week 16, week 28 and week 52 is illustrated in Table 1.
No link between the efficacy endpoints and considered variables was obtained. The impact of variables such as previous biologics, baseline PASI, and body mass index was not depicted. No discontinuations or severe safety issues associated with side effects were noted.
Therefore, risankizumab ensures a sustained clinical response over time and is effective and safe to improve moderate-to-severe forms of psoriasis.
Dermatologic Therapy
Risankizumab for the treatment of moderate-to-severe psoriasis: A multicenter, retrospective, 1 year real-life study
Giacomo Caldarola et al.
Comments (0)