Categories
Change Password!
Reset Password!


An open observational study aimed to assess the influence of etoricoxib on signs of central sensitization (CS) and pain in osteoarthritis patients.
In osteoarthritis people, etoricoxib was associated with pain improvement and reduction in severity of signs of central sensitization.
An open observational study aimed to assess the influence of etoricoxib on signs of central sensitization (CS) and pain in osteoarthritis patients.
Overall, 790 subjects with a mean age of 54.5 ± 13.0 years suffering from osteoarthritis (hip or knee) were incorporated. For two weeks, all the subjects were given etoricoxib 60 mg/day. Utilizing a numerical rating scale (NRS 0-10), an assessment of the dynamics of pain was done. Utilizing Central Sensitization Inventory (CSI), evaluation of dynamics of CS signs was done. CSI ≥40 was seen in 35.3% of subjects.
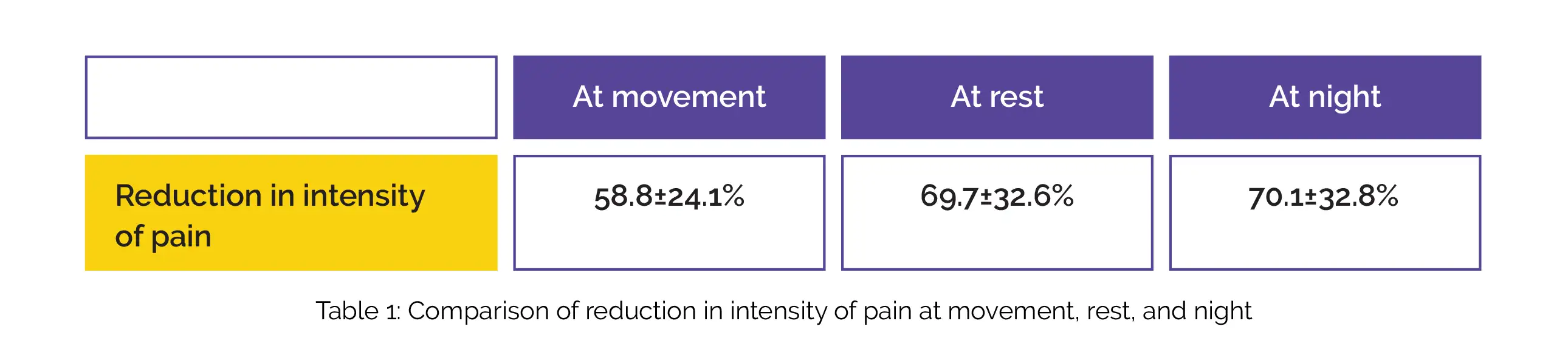
The intensity of pain at movement, at rest and at night lowered following 2 weeks of intervention with etoricoxib, as depicted in Table 1:
A reduction in CSI values by 33.1±14.5% was seen. Additionally, the number of subjects with CSI ≥40 decreased from 35.3% to 10.3%. No major complications were observed during the intervention. Notably, 5.9% of side effects, majorly arterial hypertension and dyspepsia were witnessed.
The non-steroidal anti-inflammatory drug (NSAID) etoricoxib reduced the severity of CS symptoms and pain in osteoarthritis patients, confirming the drug's key mechanism of action.
Annals of the Rheumatic Diseases
POS1134 THE EFFECT OF ETORICOXIB ON PAIN AND SIGNS OF CENTRAL SENSITIZATION IN PATIENTS WITH OSTEOARTHRITIS
E. Polishchuk et al.
Comments (0)