Categories
Change Password!
Reset Password!


This study aimed to evaluate the safety and effectiveness of re-administering Filgotinib (oral once-daily inhibitor that primarily targets Janus kinase 1), in those with ulcerative colitis who participated in the phase 2b/3 SELECTION study and its subsequent long-term extension [LTE] study, SELECTIONLTE.
In patients having ulcerative colitis, re-therapy with Filgotinib 200 mg following temporary withdrawal from therapy restores response and/or remission in the majority of induction responders within 12 weeks.
This study aimed to evaluate the safety and effectiveness of re-administering Filgotinib (oral once-daily inhibitor that primarily targets Janus kinase 1), in those with ulcerative colitis who participated in the phase 2b/3 SELECTION study and its subsequent long-term extension [LTE] study, SELECTIONLTE.
The post-hoc analysis assessed the response and remission according to the Partial Mayo Clinic Score [pMCS] in individuals who initially received induction therapy with either 200 mg [FIL200] or 100 mg [FIL100] of Filgotinib.
Participants subsequently underwent randomized treatment withdrawal [receiving a placebo] during the maintenance phase, and, in cases of disease worsening, were subsequently re-treated with open-label FIL200 during the LTE study. At LTE week 12, various factors were examined for their connection with pMCS remission. Documentation of safety outcomes was also done.
The analysis encompassed 86 patients [FIL200: n = 51; FIL100: n = 35]. The median time to disease deterioration after treatment withdrawal was reported to be 15.1 weeks (95% confidence interval [CI]: 9.1–18.7) for patients initially induced with FIL200 and 9.6 weeks [95% CI: 6.3–12.0] for those initially induced with FIL100. In both groups, 75% of people attained a pMCS response within 4–5 weeks of re-therapy. By LTE week 48, pMCS remission was attained by 45.1% of FIL200-induced patients and 51.4% of FIL100-induced patients, as shown in Figure 1:
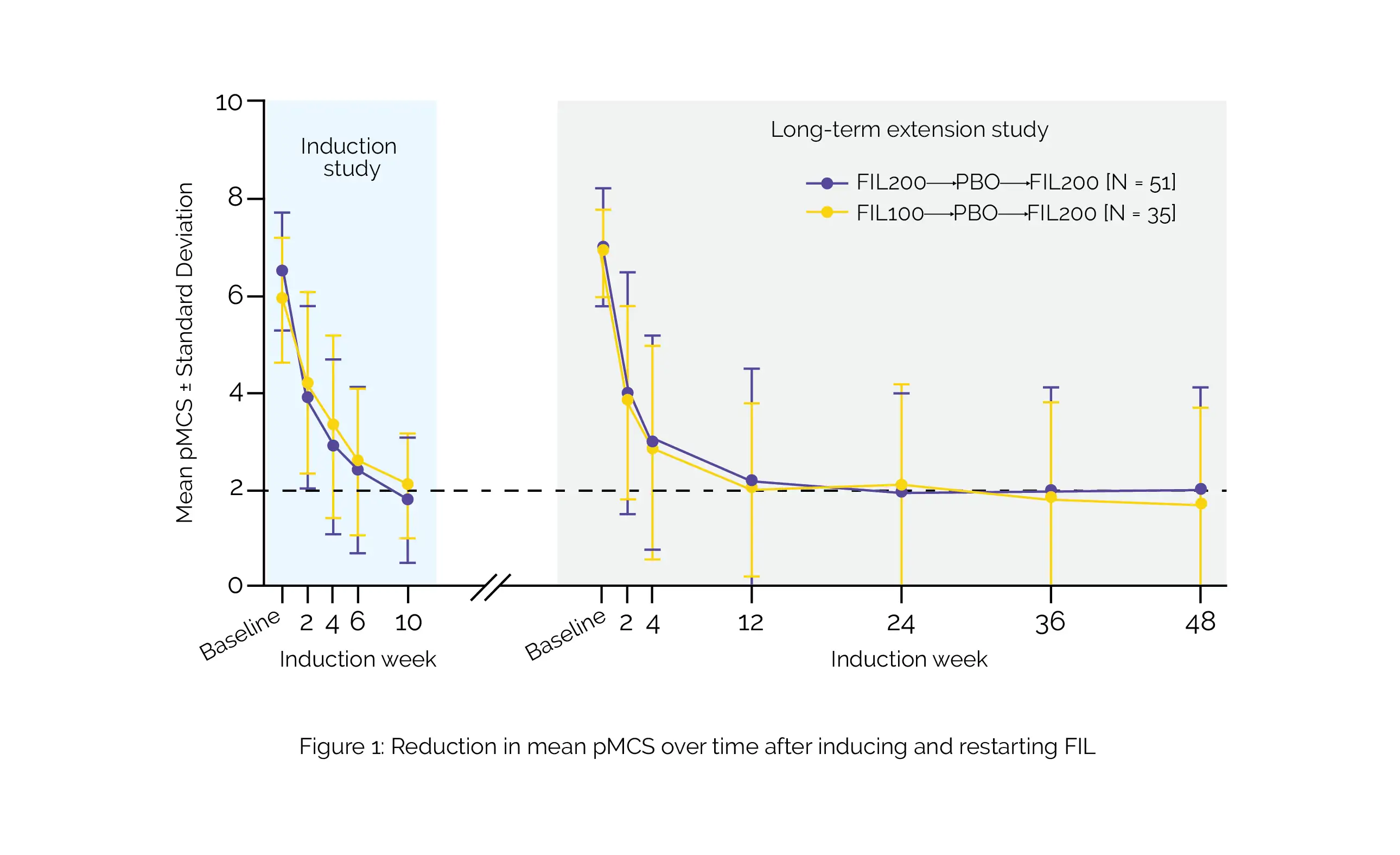
Factors independently linked with the restoration of effectiveness encompassed the absence of concomitant corticosteroid usage at the induction baseline, pMCS remission, high albumin levels, and endoscopic scores at the maintenance baseline. No novel safety concerns emerged among patients who underwent re-treatment.
In individuals who initially responded to induction therapy, re-administering FIL200 after temporary treatment discontinuation restored response and/or remission in most of the people within twelve weeks. This re-treatment approach exhibited a good tolerability profile.
Journal of Crohn's and Colitis
Withdrawal and Re-treatment with Filgotinib in Ulcerative Colitis: Post Hoc Analyses of the Phase 2b/3 SELECTION and SELECTIONLTE Studies
Séverine Vermeire et al.
Comments (0)