Categories
Change Password!
Reset Password!


A subanalysis of the PROMISE-2 trial aimed to assess the link between decrease in headache frequency and alteration in acute headache medication usage in chronic migraine sufferers.
In people with chronic migraine, Eptinezumab therapy was linked with larger reductions in headache days related to the use of acute headache medication, particularly triptans.
A subanalysis of the PROMISE-2 trial aimed to assess the link between decrease in headache frequency and alteration in acute headache medication usage in chronic migraine sufferers.
Adults with chronic migraine were enrolled and randomly assigned to receive intravenous injections of Eptinezumab (Monoclonal antibody) 100 mg, 300 mg, or a placebo once every 12 weeks for up to 2 doses. In an electronic diary, daily and event-specific records of headache and acute headache medication experiences were recorded by the patients.
Data from all days with everyday reports were incorporated. The total chronic migraine population, subjects with chronic migraine and medication-overuse headache (MOH), and subjects with MOH and chronic migraine who were ≥ 50% responders after therapy (response over weeks 1-24) were the three categories in whom changes in headache frequency and use of acute headache medication were evaluated.
Treatment was given to a total of 1072 persons with chronic migraine (Eptinezumab, n = 706; placebo, n = 366). The mean baseline acute headache medication days were 13.4, and mean baseline headache frequency was 20.5 days. Out of the 431 patients who had MOH, 225 (52.2%) showed a ≥ 50% response over the course of weeks 1-24. During weeks 1-24, proportion of days with both headache and utilization of an acute headache medication decreased by 25.1% (Eptinezumab) compared to 17.0% (placebo) in total population (n = 1072) and by 29.2% compared to 18.4% in MOH subpopulation (n = 431).
It also decreased by 38.3% compared to 31.5% in the chronic migraine with MOH population with a response of ≥ 50% (N = 225). The percentage of days with headache and the usage of triptans reduced by 9.1% (Eptinezumab) compared to 5.8% (placebo), 11.8% vs 7.2%, and 14.5% vs. 12.6%, respectively (Figure 1). Other acute headache medication categories had lower reductions.
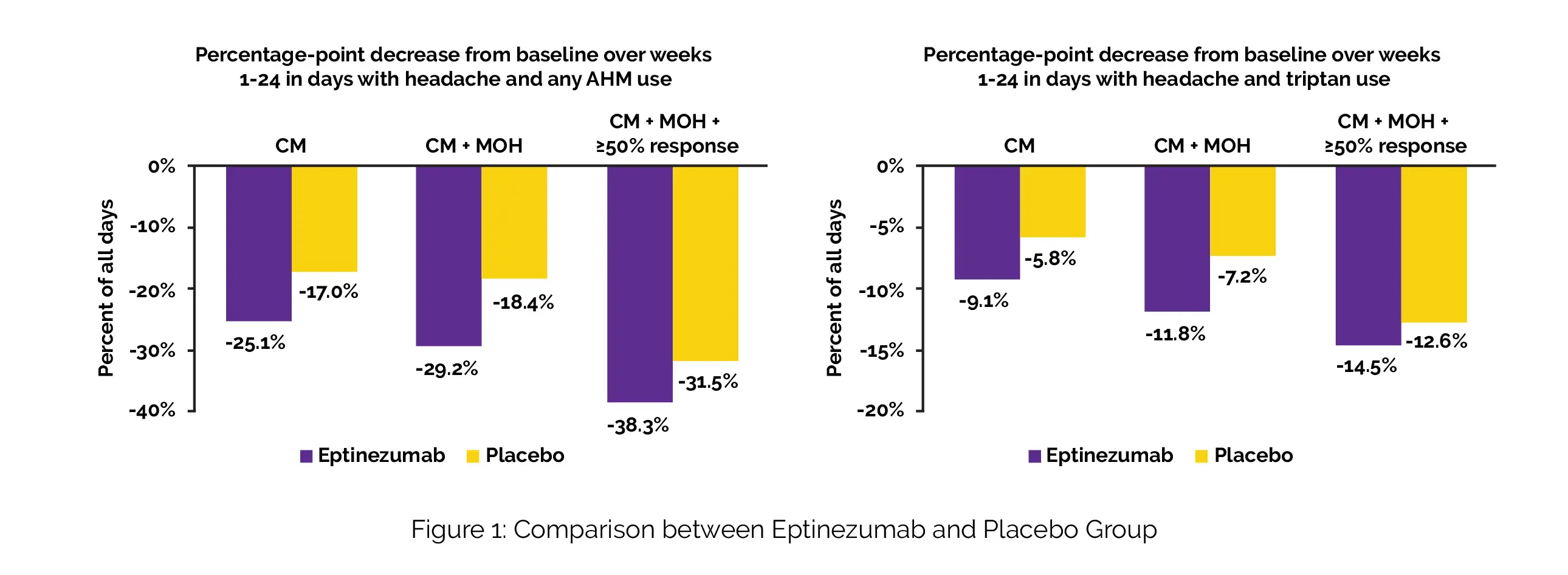
In this post hoc study, the usage of Eptinezumab in individuals with chronic migraine was linked to higher reductions in headache days when using acute headache medication overall and triptans particularly. The proportion of chronic migraine patients with MOH and ≥ 50% response experienced a larger degree of the effect.
The Journal of Headache and Pain
Quantity changes in acute headache medication use among patients with chronic migraine treated with Eptinezumab: subanalysis of the PROMISE-2 study
Robert P. Cowan et al.
Comments (0)