Categories
Change Password!
Reset Password!


A post-hoc analysis of placebo-controlled trials was conducted to assess the use of Plecanatide in adults dealing with severe constipation in the context of chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C).
In individuals experiencing severe constipation, a daily dose of 3 mg of Plecanatide is effective and well-tolerated.
A post-hoc analysis of placebo-controlled trials was conducted to assess the use of Plecanatide in adults dealing with severe constipation in the context of chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C).
Post hoc analysis was performed on data extracted from four randomized, placebo-controlled trials (two for CIC and two for IBS-C) in which participants were administered either Plecanatide at doses of 3 mg or 6 mg, or a placebo over a period of 12 weeks. Severe constipation was characterized as the absence of complete spontaneous bowel movements (CSBMs) and an average straining score of ≥3.0 on a 5-point scale (for CIC) or ≥8.0 on an 11-point scale (for IBS-C) during a 2-week screening period.
The major efficacy outcomes included two criteria:
Severe constipation was identified in 24.5% of the CIC group (646 out of 2639 individuals) and 24.2% of the IBS-C group (527 out of 2176 individuals). Both CIC and IBS-C populations exhibited significantly higher response rates with Plecanatide when compared to the placebo group (Figure 1).
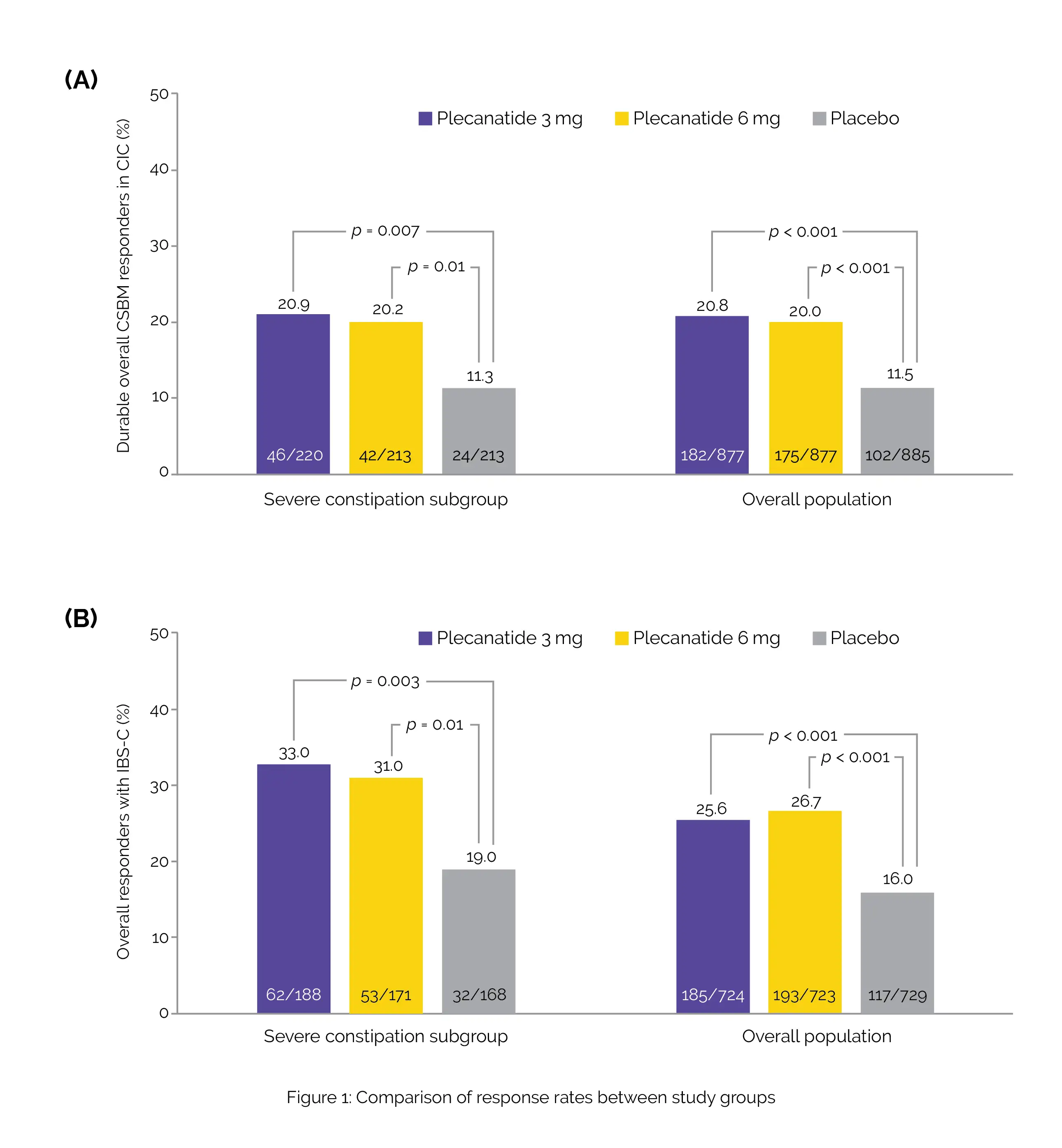
Additionally, in both populations, the time it took to experience the first CSBM was remarkably shorter for those taking Plecanatide 3 mg compared to those on placebo.
Plecanatide is a promising treatment option for severe constipation in adults battling CIC or IBS-C.
Neurogastroenterology & Motility
Plecanatide for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation: Post hoc analyses of placebo-controlled trials in adults with severe constipation
Brooks D. Cash et al.
Comments (0)