Categories
Change Password!
Reset Password!


A randomized, open-label trial aimed to assess the safety and effectiveness of Mirogabalin as an adjuvant to NSAIDs for lumbar spinal stenosis (LSS) patients than NSAIDs alone.
In comparison to NSAIDs alone, Mirogabalin + NSAIDs led to better improvements in quality of life, pain intensity, and was well-tolerated in patients with lumbar spinal stenosis.
A randomized, open-label trial aimed to assess the safety and effectiveness of Mirogabalin as an adjuvant to NSAIDs for lumbar spinal stenosis (LSS) patients than NSAIDs alone.
In MiroTAS study, the total number of eligible patients that were enrolled was 220. NSAIDs monotherapy and Mirogabalin + NSAIDs were given to patients in a 1:1 ratio. Mirogabalin dosages were determined on the basis of kidney function [creatinine clearance (CrCL) ≥ 60 mL/min, 5 mg two times a day (BID) in weeks 1-2, 10 mg twice daily in weeks 3-4, and 15 or 10 mg twice daily post week 5; CrCL 30 to less than 60 mL/min, 2.5 mg twice daily weeks 1-2, 5 mg twice daily weeks 3-4, and 7.5 or 5 mg twice daily post week 5].
The difference between the baseline and Week 12 visual analog scale (VAS) scores for leg pain was the major outcome. Safety, and quality of life, as measured by Patient Global Impression of Change (PGIC) at 12th week and EuroQol five-dimensional descriptive system (EQ-5D-5L) at baseline and 12th week were the secondary outcomes. A linear mixed model for repeated measurements was used to determine the alteration in the VAS score at week 12. Adverse drug reactions and treatment-emergent adverse events (TEAEs) served as the safety outcomes.
At enrollment, the percentage of subjects with VAS score ≥ 60 (53.6% vs. 52.9%), mean VAS scores (63.8 vs. 62.8 mm), mean CrCL (81.5 vs. 70.7 mL/min), mean body weight (63.9 vs. 62.0 kg), proportions of female patients (54.5% vs. 49.0%), proportions of patients with CrCL 30 to < 60 mL/min (27.3% vs. 33.7%), and mean ages (67.8 vs. 70.9 years) were similar in both the groups.
For Mirogabalin + NSAIDs and NSAIDs alone group, 6.8 and 7.8 were the Spine pain DETECT questionnaire (SPDQ) scores correspondingly, and the median LSS durations were 9.0 and 11.0 months respectively. In the Mirogabalin + NSAIDs group, the least square (LS) mean alteration in VAS score from baseline to week 12 was higher when compared to the NSAIDs group, as shown in Table 1:
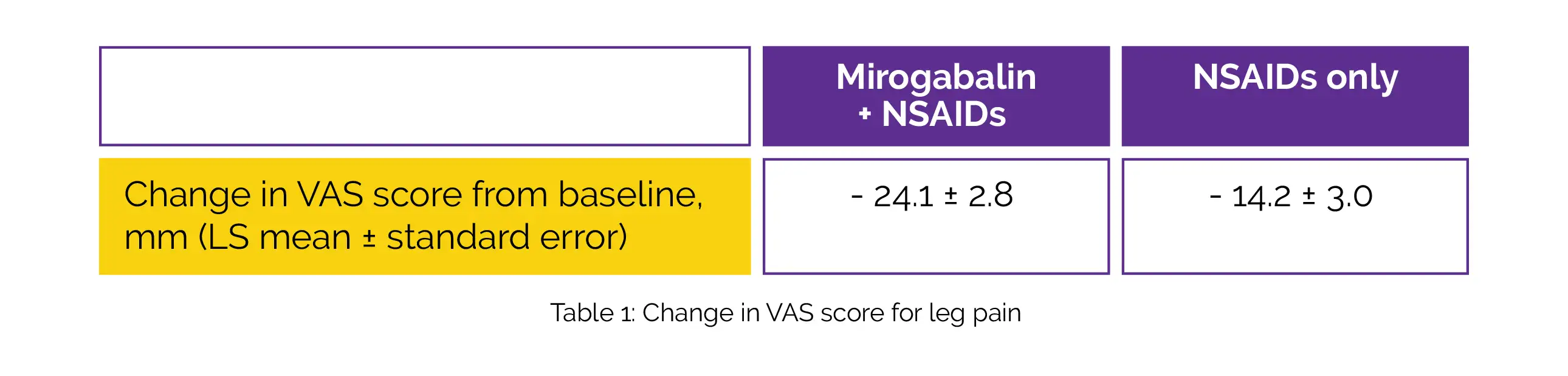
The LS mean difference was -9.9. The Mirogabalin + NSAIDs group considerably outperformed the NSAIDs group in terms of improvement in EQ-5D-5L score at week 12 [mean difference, 0.0529]. The percentage of subjects with PGIC scores ≤ 3 and ≤ 2 in the Mirogabalin + NSAIDs group compared to the NSAIDs group were greater at week 12, as shown in Table 2:
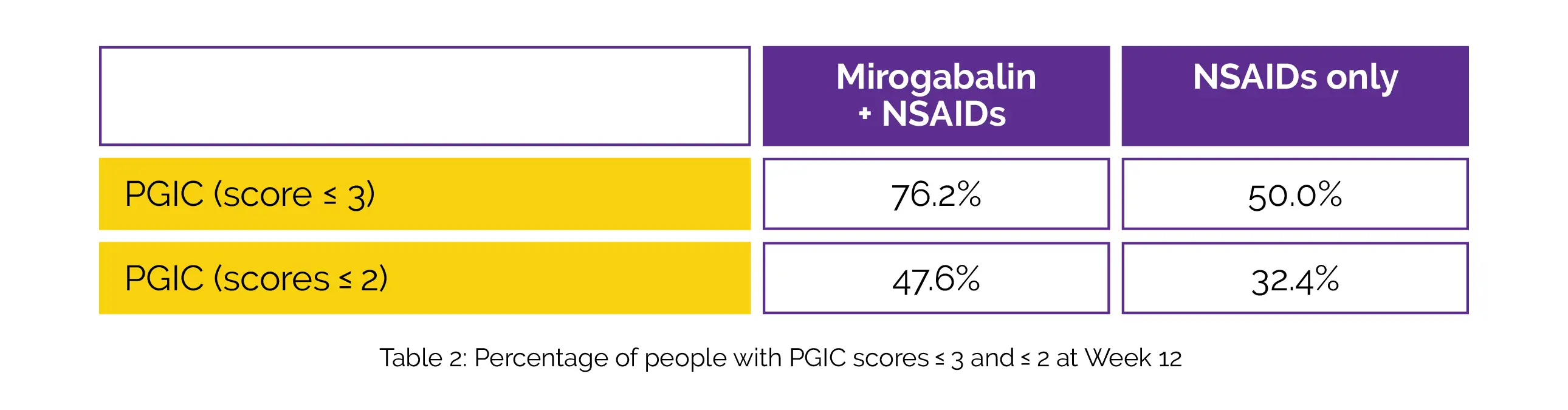
The most frequent TEAEs in the Mirogabalin + NSAID group were dizziness (25.5%) and somnolence (30.0%), with frequencies of TEAEs and adverse drug reactions being 60.9% and 57.3%, respectively.
Adding Mirogabalin to NSAIDs effectively reduced peripheral neuropathic pain related to LSS while posing no additional safety issues.
Pain and Therapy
Efficacy and Safety of Add-on Mirogabalin to NSAIDs in Lumbar Spinal Stenosis with Peripheral Neuropathic Pain: A Randomized, Open-Label Study
Takuya Nikaido et al.
Comments (0)