Categories
Change Password!
Reset Password!


This randomized controlled trial examined Lubiprostone's effectiveness and safety in tackling pediatric functional constipation.
Using Lubiprostone is an effective, safe, and well-tolerated strategy for childhood and adolescent functional constipation. Its positive effects are sustained even after treatment discontinuation.
This randomized controlled trial examined Lubiprostone's effectiveness and safety in tackling pediatric functional constipation.
This study, conducted as a single-blinded study, incorporated 280 subjects aged 8-18 with functional constipation. Participants (adolescents and children) were randomly segregated to receive either a weight-based dose of Lubiprostone (n = 140) or conventional laxatives (n = 140) such as sodium picosulfate, bisacodyl, or lactulose for a duration of twelve weeks. This was followed by a 4-week post-observation period.
Remarkable improvements were witnessed in constipation among patients in the Lubiprostone group, as opposed to the conventional therapy group (p < 0.001), as depicted in Table 1 below:
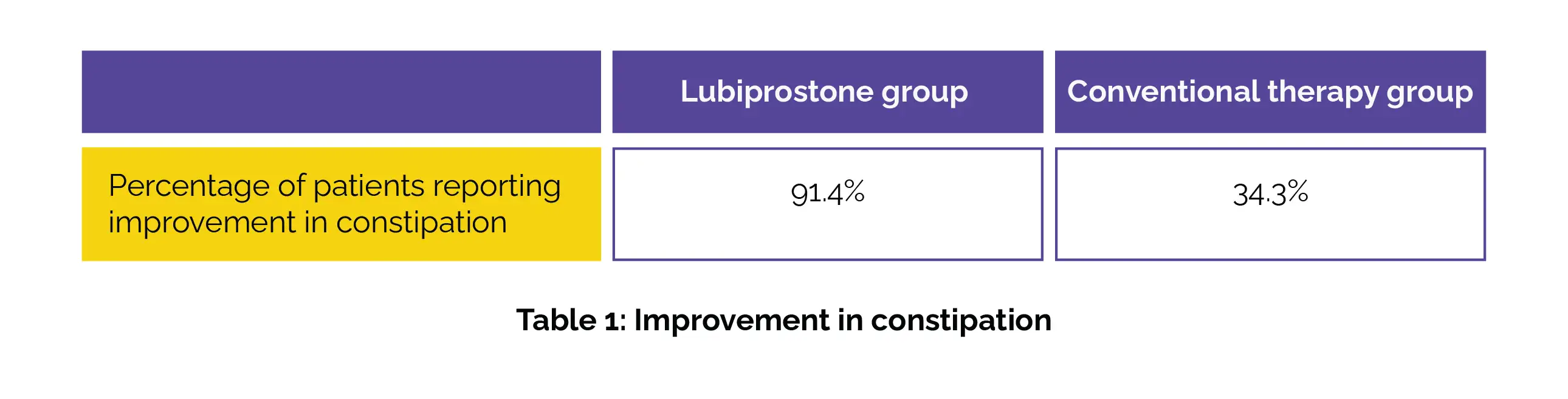
This betterment was sustained even after the discontinuation of therapy. Additionally, one-quarter of patients in the Lubiprostone group had their first spontaneous bowel movement within 48 hours of commencing the medication. Throughout the last four weeks of therapy and the four-week follow-up period, 75.7% of the Lubiprostone group maintained a Bristol stool form of 3 or 4, compared to 35.7% in the conventional therapy group (p < 0.001).
No severe adverse reactions to Lubiprostone were documented. Also, there were no instances of treatment discontinuation owing to adverse effects. In the Lubiprostone group, the most common side effects were mild and self-limiting headache and colicky abdominal pain.
Lubiprostone proved to be an efficient, safe, and well-tolerated pharmacotherapy for pediatric functional constipation, suggesting a potential shift in the approach to managing this ailment in younger age groups.
Journal of Pediatric Gastroenterology and Nutrition
Efficacy of lubiprostone for functional constipation treatment in adolescents and children: Randomized controlled trial
Engy S Elkaragy et al.
Comments (0)