Categories
Change Password!
Reset Password!


This longitudinal, prospective, cohort study was carried out to investigate the long-term benefit (efficacy and safety) of UC-MSC to treat severely infected COVID-19 patients.
In people with severe COVID-19, administration of umbilical cord-derived mesenchymal stem cell (UC-MSC) has good tolerability and offers a long-term benefit in the improvement of lung lesions and symptoms.
This longitudinal, prospective, cohort study was carried out to investigate the long-term benefit (efficacy and safety) of UC-MSC to treat severely infected COVID-19 patients.
In this study, a total of 100 subjects enrolled in the phase 2 trial were followed up for one year at three-month intervals. Participants were randomized to get either UC-MSC (n = 65) or placebo (n = 35) along with standard care.
Using high-resolution computed tomography (CT), the changed proportion of whole-lung lesion volumes (major endpoint) was estimated. Additional imaging outcomes like lung function, plasma biomarkers, 6 min walking distance (6-MWD) were also analyzed. Side effects were also monitored.
On day 10 after administration of UC-MSC, the whole-lung lesion volume was improved with a difference of −10.8% when compared to placebo. At every follow-up point, the prevalence of symptoms and the percentage of solid component lesion volume decreased in UC-MSC recipients in comparison with placebo recipients.
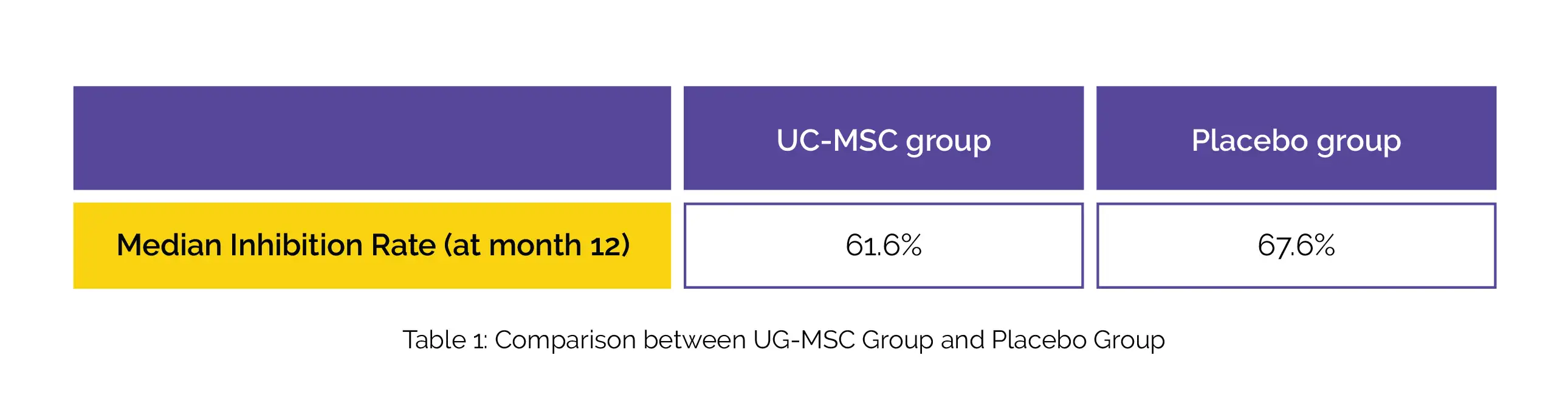
At month 12, no patient in the placebo group and 17.9% (10/56) patients in the MSC group exhibited normal CT images. At the 1-year follow-up, no inter-group difference was witnessed in terms of adverse effects. At month 12, no inter-group difference in tumor markers was noted. As found, all the neutralizing antibodies were positive. At month 12, both groups illustrated similar median inhibition rate of neutralizing antibodies, as shown in Table 1:
UC-MSC improves the recovery of lung lesions and symptoms in coronavirus-infected patients. Thus, the use of human mesenchymal stem cells as adjunctive therapy for the management of severe COVID-19 is a feasible therapeutic choice.
THE LANCET
Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial
Lei Shi et al.
Comments (0)