Categories
Change Password!
Reset Password!


In dentistry, pain management is one of the most crucial aspects to be addressed while developing treatment plans.
Oral Ketorolac can be effectively used as a premedication for endodontic management of irreversible pulpitis prior to inferior alveolar nerve block (IANB)
In dentistry, pain management is one of the most crucial aspects to be addressed while developing treatment plans. To produce substantial anesthesia during endodontic treatment, IANB has become a standard procedure. But, IANB alone has been noted to have a success rate that ranges from 15 to 57%. As of late, scavenging of reactive intracellular products has been linked to the anti-inflammatory effect of non-steroidal anti-inflammatory drugs (NSAIDs), which work by inhibiting cyclooxygenase.
In numerous randomized controlled trials, the use of NSAIDs and opioid analgesics for inflammation of dental pulp prior to local anesthesia has been investigated. The NSAID Ketorolac has positive effects in patients with excessive or severe inflammation and can even be used as an opioid-sparing analgesic. When treating patients with acute pain in pre-hospital situations, parenteral Ketorolac has been recommended as an acceptable analgesic. People suffering from irreversible pulpitis experience moderate to severe odontogenic pain, which makes IANB less likely to be successful.
RATIONALE BEHIND RESEARCH
According to a study, pulpitis patients who do not experience pain have a higher success rate with IANB than those who do experience pain. As a result, numerous researchers have experimented with Ketorolac as a pre-anesthetic drug to increase IANB success. Till date, no comprehensive compilation of such data has been made in order to compare the relative effectiveness of Ketorolac to other pre-anesthetic medications.
OBJECTIVE
This study was performed to examine the anesthetic effectiveness of IANB following oral Ketorolac pre-medication for endodontic therapy of tooth with irreversible pulpitis.
Literature search
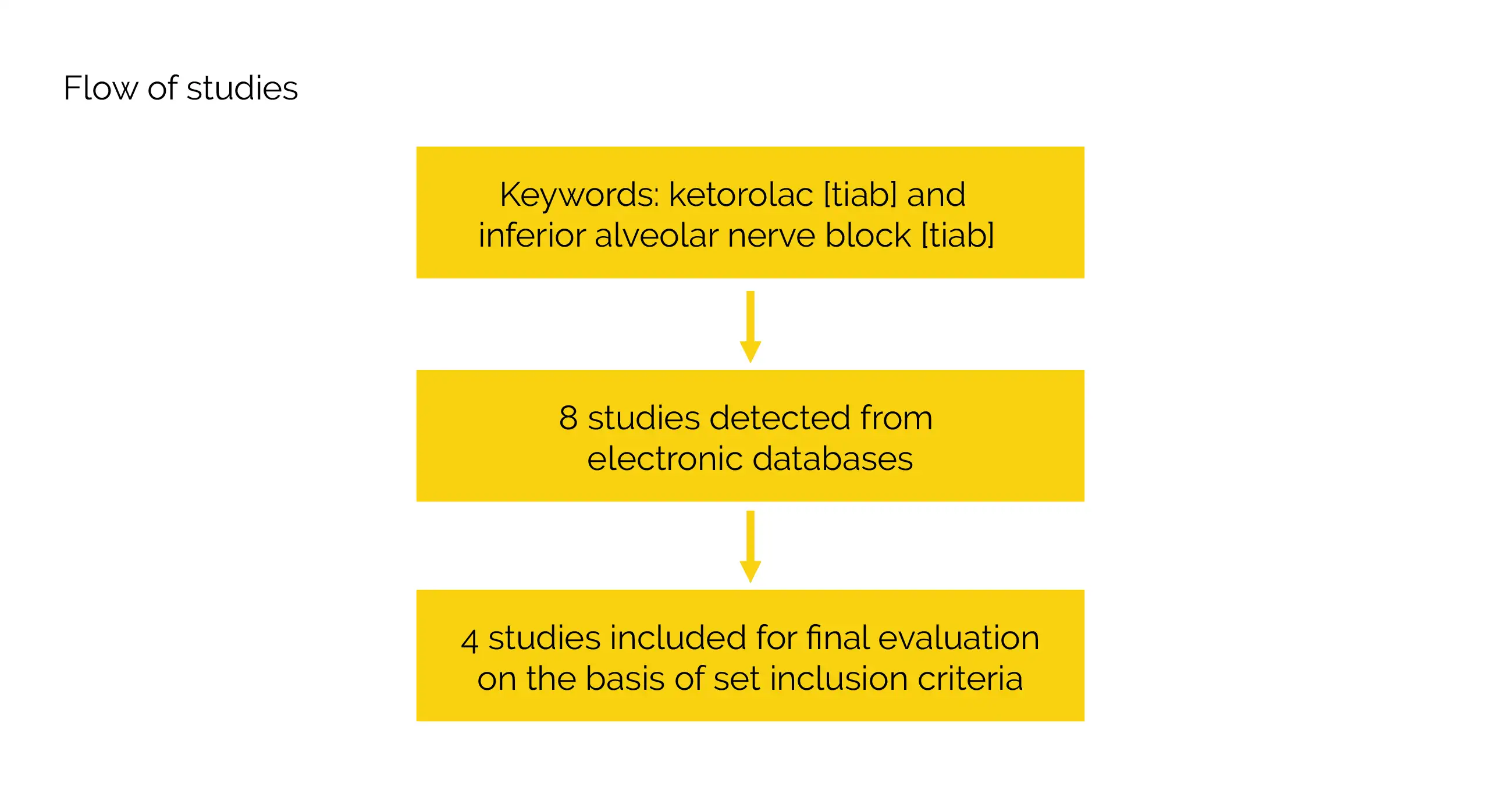
Ketorolac [tiab] and inferior alveolar nerve block [tiab] were used as keywords in a comprehensive literature search that was carried out with the aid of Database of Abstracts of Reviews of Effects (DARE), Medline/PubMed, and Cochrane central register of clinical trials (CENTRAL). The references from appropriate studies were manually searched as an additional supplement to this search. Research that was published in English language was incorporated.
Inclusion criteria
Studies were included if:
Study selection and Data extraction
Screening of data followed by identification of abstracts for possible inclusion was done independently by 2 authors. For every study that qualified, full-text versions were chosen. The trial site, year, trial techniques, participants, therapies, and outcomes were extracted using a pre-tested information extraction form. The RevMan 5.0 programme was used to analyze the retrieved data in non-Cochrane mode.
Data and Statistical Analysis
The percent difference was evaluated between experimental (Ketorolac ) and control (any other drug or placebo) groups. Final evaluation was based on mean difference in percent and percent standard error. Relative risks [95% confidence interval (CI)] for IANB success were calculated.
Utilizing visual Forest plot, I2 statistics, where more than 50% was deemed to have moderate to severe variation, and the Chi-square tests with a statistical P-value < 0.10 to demonstrate statistical significance, the heterogeneity between the studies was evaluated. In cases with moderate heterogeneity, random effect models were applied.
Risk of Bias and Quality assessment
Cochrane collaboration’s tool assessed the risk of bias of eligible trials. Allotment concealment, sequence generation, selective outcome reporting, blinding (of volunteers, staff, and outcome assessors), incomplete outcome data, and other potential sources of bias were the areas evaluated to determine whether trials had taken the necessary precautions to lower the risk of bias. Notably, ‘low’, ‘high’, or ‘unclear’ were the parameters of risk of bias. There were not enough studies to check for publication bias.
Study outcomes
Outcomes
Study and participant characteristics:
Effect of intervention on the outcome:
This systematic review intended to determine the anesthetic efficacy of oral Ketorolac pre-medication in combination with traditional IANB using lignocaine. Prior to performing endodontic therapy for teeth having irreversible pulpitis, an IANB therapy with lignocaine and adrenaline has been commonly used to achieve a high level of analgesia. But, 90% of the practitioners face difficulties in attaining profound analgesia. Particularly, in teeth exhibiting irreversible pulpitis, an increased incidence of failures has been documented to occur with IANB.
Anxiety and panic, accessory innervation and concentration, as well as the amount of local anesthetic and vasoconstrictor are a few of the stated causes of this failure. Taking into account the failure rates, the studies focused on employing NSAIDs and steroids as pre-medication and discovered the anesthetic effectiveness of IANB after pre-medication. Lapidius et al. thoroughly analyzed the impact of pre-medication on IANB. But, not all studies were incorporated in the systematic assessment. It has been claimed that pre-operative medicine can improve the effectiveness of IANB.
In numerous randomized controlled trials, NSAIDs like ibuprofen, Ketorolac, diclofenac, and steroids such as dexamethasone have been tested. Due to Ketorolac's benefits over other NSAIDs, it has become more popular. These encompass single dosage administration, less likely combination interactions when co-administered with other medications, less likely bleeding time changes, and less likely to trigger acute renal failure in people with pre-existing kidney damage.
This comprehensive review and meta-analysis determined the anesthetic efficiency of IANB following pre-medication with Ketorolac, taking into account the merits of Ketorolac over other NSAIDs. Besides Ketorolac, other NSAIDs may include ibuprofen and acetaminophen (used together). Prednisolone exhibited a superior effect over Ketorolac and placebo in a recent trial by Praveen et al. that compared the two drugs. Besides oral use, Ketorolac was also proven to work well when administered buccally. Contrarily, combining adjunct Ketorolac plus nitrous oxide does not seem to have any additional analgesic effects in persons with symptomatic pulpitis.
Ketorolac oral pre-medication was found to have a statistically significant impact on success rates of an IANB in people with irreversible pulpitis in the four trials that were part of the systematic review and meta-analysis. But, the VAS-measured pain levels did not considerably differ. Further, additional long-term randomized trials are required to substantiate Ketorolac's effectiveness.
EMBASE could not be explored for appropriate studies
The Open Dentistry Journal
Oral Ketorolac with Inferior Alveolar Nerve Block for Irreversible Pulpitis: A Systematic Review and Meta-analysis
Gowri Sivaramakrishnan et al.
Comments (0)