Categories
Change Password!
Reset Password!


In clinical practice, onychomycosis (OM) stands out as the most prevalent nail ailment resulting from fungal organisms such as yeast, non-dermatophytes, and dermatophytes.
In onychomycosis patients with poor prognostic factors or those who do not respond to monotherapy, combination therapy must be reserved as a second-line therapeutic option.
In clinical practice, onychomycosis (OM) stands out as the most prevalent nail ailment resulting from fungal organisms such as yeast, non-dermatophytes, and dermatophytes. Clinically, it is characterized by subungual hyperkeratosis, nail plate onycholysis, and thickening. This results in significant psycho-social, aesthetic, and physical consequences. Its significance goes beyond cosmetic concerns, frequently leading to pain, challenges in walking, performing daily tasks, and adversely affecting overall quality of life.
Currently, the United States Food and Drug Administration (US-FDA) has approved oral terbinafine, griseofulvin, and itraconazole, along with topical ciclopirox, tavaborole, and efinaconazole for OM management. The off-label use of oral fluconazole is common in the treatment of this condition. In a systematic review and meta-analysis that encompassed 26 randomized controlled trials (RCTs) examining systemic monotherapy for toenail OM, all monotherapy treatments illustrated a considerably higher odds ratio (OR) for attaining mycological cure compared to placebo.
The treatment of OM poses challenges and is tailored to individual factors such as infecting organisms, comorbidities, ailment severity, and takes into account cost, drug interactions, and potential adverse events of medications. OM monotherapy has certain drawbacks, including the risk of potential antifungal resistance and the challenge of attaining sufficiently high concentrations of biologically effective drugs in affected nails, especially in severe cases of OM. In a blinded, five-year follow-up investigation with participants who attained mycological cure 12 months post oral itraconazole or terbinafine monotherapy, 23% and 53% of subjects, respectively, experienced either mycological relapse or reinfection.
Hence, there is a necessity for studies incorporating extended follow-up periods to monitor recurrences post-complete cures, aiming to refine treatment regimens and deter relapses. Combinations of devices, topical treatments, and oral medications are being explored, particularly in instances where poor responses to monotherapy are anticipated, there is > 50–60% nail involvement, or > 3 nails are affected. Concurrent combination therapy is advised for volunteers at a higher risk of treatment failure, such as those with underlying comorbidities like diabetes.
On the other hand, sequential therapy is advocated for patients showing inadequate responses to the initial therapy. The rationale behind combination therapy lies in the potential for antimicrobial synergy, broader antifungal coverage with enhanced fungicidal activity, reduced resistance, and improved clinical outcomes, especially when employing drugs having diverse mechanisms of action or administration routes.
RATIONALE BEHIND RESEARCH
Combination therapy has attracted substantial attention due to its potential for synergistic effects and the prevention of antifungal resistance. However, it has not undergone a thorough investigation. There is a scarcity of research on combined antifungal therapy for OM, and the latest reviews available span from 1999 to 2006. Therefore, this study was conducted.
OBJECTIVE
The aim of this systematic review was to assess clinical trials comparing combination therapy to monotherapy in treating OM, with the goal of offering insights for clinical management.
Literature search
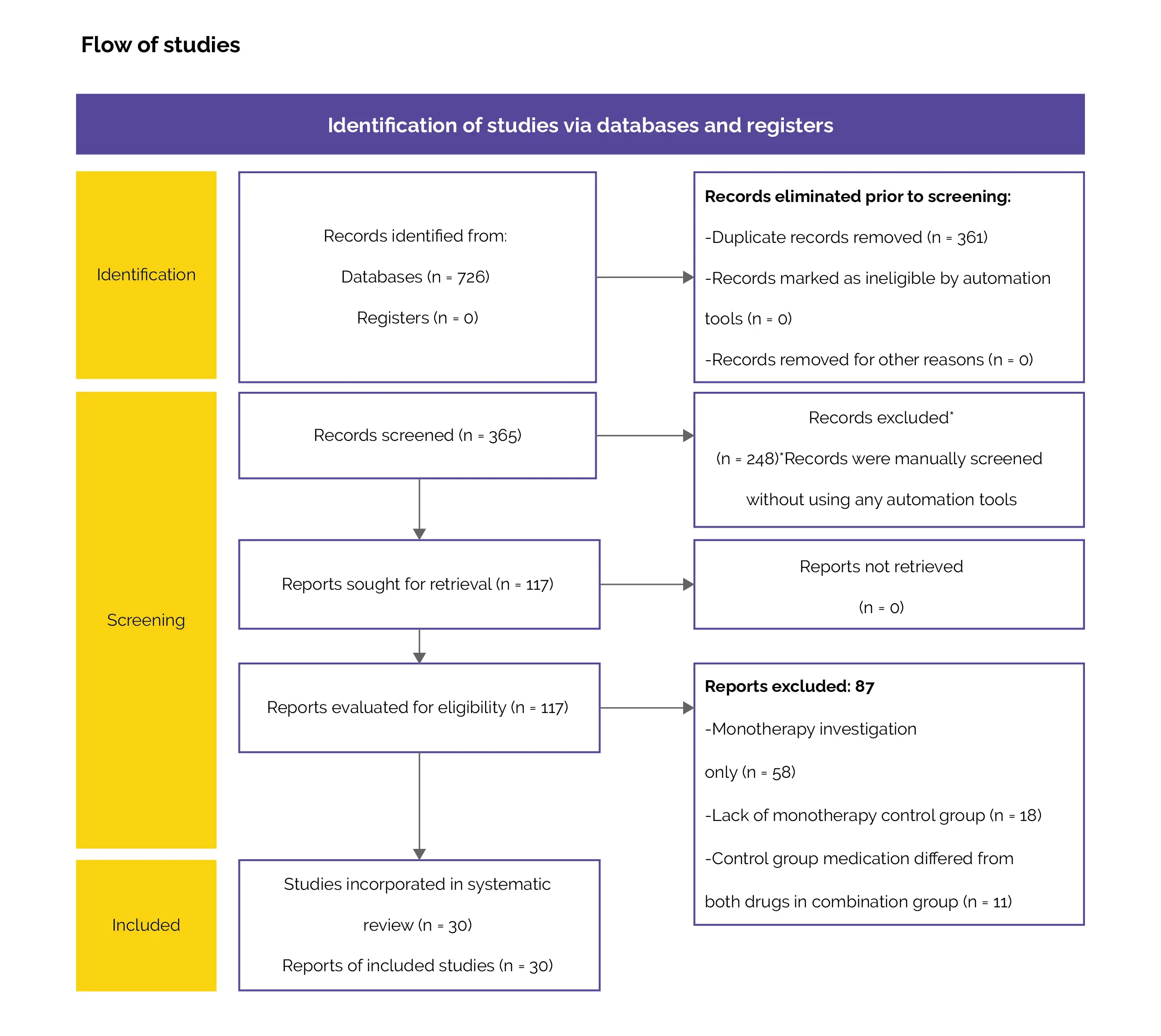
A search was conducted on the Web of Science, Scopus, and PubMed databases for articles related to OM combination therapy. The search terminologies employed were "onychomycosis treatment", "onychomycosis therapy", and "onychomycosis combination therapy".
Inclusion criteria
This systematic review incorporated studies that met the following criteria:
Exclusion criteria
Excluded from consideration were studies investigating monotherapy treatment regimens, non-clinical trials, non-randomized trials, non-English studies, and duplicates.
Additionally, studies that investigated diagnoses beyond OM (such as tinea pedis), did not incorporate monotherapy control arms, or utilized control group medicines that varied from both drugs in the combination arm were also eliminated.
Study selection and Data extraction
Two authors independently evaluated articles by screening their abstracts. Subsequently, both authors individually examined the full-text articles to determine eligibility and extracted relevant data from qualifying studies. The extracted information from each study encompassed outcomes such as adverse effects, treatment success rate, treatment protocol, and the number of subjects. Any unavailable data was explicitly indicated as "N/A".
Data and Statistical Analysis
N/A
Risk of Bias and Quality assessment
N/A
Study outcomes
Treatment success rate (mycological, complete, and clinical cure rates) and adverse effects were the outcomes evaluated.
Outcomes
Study and participant characteristics:
Study quality:
Seven studies focusing on medication produced inconsistent results, showing similar quality across all the studies.
Effect of intervention on the outcome:
The findings of this study revealed that combination therapy illustrated efficacy in relieving OM in over half of the trials, with minimal reported adverse events. However, a total of seven studies reported conflicting results, and in cases where monotherapy displayed superiority, the significance diminished later or was witnessed only in longer treatment groups.(e.g., oral terbinafine for 12 weeks versus 6 weeks). The trials for medicine-only combination therapy had relatively short durations, averaging 46.1 weeks (Standard deviation [SD]: 21.5; range: 24–78.2 weeks).
On the contrary, pivotal RCTs for topical efinaconazole, oral itraconazole, and oral terbinafine featured extended follow-up durations: 62.1 weeks (SD: 28.7; range: 40–104.3), 66.4 weeks (SD: 55.8; range: 19–252), and 69.0 weeks (SD: 49.1; range: 36–252), respectively. Overall, trials for medicine-only combination therapy were less rigorously designed than pivotal monotherapy trials, requiring cautious interpretation. Seven studies based on medication interventions revealed conflicting results, with a comparable level of quality among them.
Two studies presented divergent results regarding the outcomes of oral itraconazole and topical amorolfine therapy. The first study, conducted across multiple centers, included 131 volunteers with a 5.5-month follow-up, while the second study, a single-center trial, involved 90 volunteers with a 9-month follow-up. Moreover, three studies reported contradictory findings regarding the outcomes of combining oral terbinafine with topical ciclopirox therapy. The first study, which was single-center and non-blinded, consisted of 80 subjects with a 9-month follow-up. The second study, a multi-center, single-blinded trial, included 73 subjects with an 11-month follow-up. The third study, a single-center trial with single-blinding, comprised 96 patients with an 8.3-month follow-up.
In two studies evaluating the effectiveness of combining oral itraconazole and oral terbinafine therapy, conflicting results were observed. The first study, encompassing 190 patients across multiple centers, featured a 16.6-month follow-up and single-blinding. In contrast, the second study involved 106 patients in multicenter settings and employed single-blinding. As these conflicting trials shared similar quality standards and lacked a definitive demonstration of the superiority of combination over monotherapy treatments, it underscores the need for extensive, multicenter, double-blinded trials with ample follow-up to ascertain the effectiveness of combination therapy in tackling OM.
Three studies focusing on medication-only treatments, which encompassed cost information, indicated that combination therapy was more cost-effective than monotherapy when considering both efficiency and duration of usage. However, it's pivotal to note that this conclusion may not be applicable to combination oral therapy involving novel topical agents. An examination of data extracted from the National Average Drug Acquisition Cost Medicaid Pharmacy Pricing database spanning 2013–2018 indicated an annualized decrease in inflation-adjusted costs of -3.4% for terbinafine and -18.2% for generic oral itraconazole.
In contrast, brand name medications such as Kerydin, Jublia, and Diflucan witnessed escalation of 3.7%, 4.5%, and 17.2%, respectively. This information implies that using branded topical and oral antifungal agents can be expensive, and incorporating them into combination treatment plans may trigger higher healthcare costs. In a study assessing the cost of a 48-week treatment course of topical efinaconazole 10% solution for a single toenail ($8057), the expenses were nearly 35 and 12 times higher than the estimated costs for a three-month treatment with oral terbinafine (250 mg/day, $233) and oral itraconazole (200 mg/day, $683), respectively.
It is fundamental to emphasize that these comparisons do not take into account the relative effectiveness. The evaluation of Medicare provider utilization and payment data (part D) from 2013 to 2018 revealed that the total costs for topical efinaconazole escalated annually by 3091%, and costs per supply day rose by 144%. In contrast, topical tavaborole costs experienced a 12.5% decline, while costs per supply day elevated by 42.4%. This emphasizes the necessity for additional research to gauge the cost-effectiveness of combination treatments compared to monotherapy. The findings revealed that almost all studies examining combined procedural and medication therapy illustrated substantial benefits in comparison with monotherapy.
However, the trials had relatively short durations, averaging 32.5 weeks (SD: 14.3; range: 13.04–62.0 weeks). RCTs focusing on photodynamic and laser monotherapies exhibited a similar average follow-up duration of 35.3 weeks (SD: 11.5; range: 24.0–52.0 weeks). This suggests a common inadequacy of extended follow-up periods in procedural studies for assessing the efficacy of OM management. Additionally, in the review, certain monotherapy and combination approaches did not show efficiency for severe cases of OM. In a review of 24 laser trials targeting OM, there was a dearth of evidence endorsing the efficacy of lasers in treating the condition.
Approximately 30% and 20% of trials included detailed descriptions of their allocation modes or incorporated blinding in their experimental designs, respectively. Additionally, a systematic review of 25 RCTs exploring laser monotherapy for toenail OM revealed that mycological cure was assessed in only one study, with no reported instances of complete cure across all studies. The alterations in the mean OSI from baseline were minimal, ranging from -3.6 to +1.4. Moreover, the efficacy of both control and treatment groups was comparable, failing to demonstrate improvements in the "temporary increase of clear nail", which is the endpoint approved by the US-FDA.
The methodology employed in the present laser trials, whether focused on monotherapy or combination treatments as outlined in the review, is deemed insufficient. Consequently, it is imperative to substantiate the efficacy of laser monotherapy through rigorous RCTs prior to contemplating its usage in combination with other OM medications. Notably, the cost factor was not disclosed in any of the medication and procedural combination trials assessed in the review. Laser treatments are known to be costly, not covered by insurance, and typically necessitate multiple monthly sessions.
Considering the relatively lower effectiveness and higher cost of laser therapies compared to topical or oral treatments, caution is advised against combining lasers with antifungal medications until more robust RCTs are carried out. These trials ought to exhibit superior efficacy while maintaining favorable cost-benefit profiles. Notably, combination therapy generally depicted good tolerability in studies focusing solely on medication. In a comprehensive review and meta-analysis comprising 26 trials exploring monotherapy for toenail OM, the OR for adverse events in any group did not show a significant difference from the placebo (except in the instance of efinaconazole 10% solution (OR 1.28) for transient application-site reactions).
Hence, as monotherapy demonstrates good tolerability in the treatment of OM, it is recommended to opt for single-medication therapy to minimize adverse effects. In studies involving both procedures and medications, a greater occurrence of adverse events was witnessed with combination therapy compared to monotherapy. Nevertheless, it is crucial to acknowledge that the sample sizes in all three studies were small, and in each instance, monotherapy consisted of either a topical treatment or a placebo rather than a procedural intervention. Consequently, uncertainty persists regarding whether the adverse events observed in the combination group can be attributed to the procedure itself (such as skin irritation from laser therapy) or arose from the combination of therapies.
In a systematic review encompassing 35 trials with 1723 subjects and 4278 nails affected by OM, the majority of participants indicated a mild-to-moderate burning sensation during laser therapy, with a few even noting instances of bleeding. In pivotal RCTs exploring the efficacy of oral terbinafine as a standalone therapy, adverse effects were temporary, of mild to moderate intensity, and did not substantially differ from those observed in the placebo groups. Notably, there were no reported abnormalities in laboratory tests. As per the terbinafine package insert, only 3.3% of patients experienced liver enzyme abnormalities, and taste disturbances were reported in 2.8% of cases.
The discontinuation rate for both issues was low, standing at 0.2%. Conversely, monotherapy with oral itraconazole is generally well-tolerated, and the most commonly reported adverse events are mild and temporary, including gastrointestinal discomfort and headaches. In a phase-III RCT, the safety profiles were comparable between the placebo and itraconazole treatment groups. In two multicenter phase-III RCTs evaluating the effectiveness of topical efinaconazole as a standalone treatment, the incidence of adverse events was comparable between the treatment and vehicle (control) groups in both studies. The majority of these events were of moderate or mild intensity, and there were minimal instances of therapy-related discontinuation.
Furthermore, there were no clinically significant alterations in laboratory values or vital signs from the baseline. Therefore, when employed as monotherapy, FDA-approved treatments for OM exhibit minimal side effects, none of which pose a life-threatening risk, and instances of medication discontinuation are rare. Nevertheless, it's crucial to highlight that oral itraconazole and fluconazole are contraindicated when used alongside specific medications. Moreover, oral itraconazole, fluconazole, and terbinafine have the potential to influence the plasma concentration of certain drugs.
Given the notable occurrence of drug interactions between oral therapies for OM and other medications, the commencement of oral combination therapy necessitates vigilant monitoring of patients concurrently taking multiple medications. In such cases, adjustments or reductions in medication dosage are more likely compared to patients undergoing monotherapy treatment. In a meta-analysis of 26 RCTs examining systemic monotherapy for toenail OM, all treatments demonstrated a significantly higher OR for achieving mycological cure compared to the placebo. Notably, continuous itraconazole at 200 mg (OR: 18.61) and continuous terbinafine at 250 mg (OR: 16.41) emerged as the most effective treatments.
Consequently, monotherapy proves to be an effective approach in combatting OM. Therefore, when considering the analysis of combination therapy for OM, it is recommended to view monotherapy as the primary treatment option before considering combination therapy. Indicators of a less favorable prognosis for treating OM encompass patient characteristics such as older age or a personal history of OM, comorbidities like immunosuppression, peripheral vascular disease, or uncontrolled diabetes mellitus, specific nail characteristics (prior nail trauma, proximal subungual OM, dermatophytoma, severe onycholysis), and the type of infecting organism (mixed fungal infection, non-dermatophytes, yeasts).
Specific genetic factors might make individuals more susceptible to mucocutaneous fungal infections. For instance, mutations in Tyr238X dectin-1 or caspase recruitment domain-containing protein-9 can result in impaired recognition of β-glucan and reduced cytokine responses. Additionally, abnormalities in major histocompatibility complexes may hinder the initial recognition of fungi and impede the activation of T-cells. Modifications in intercellular adhesion molecule-1 might hinder the migration of immune cells to infected tissues, and heightened levels of T-regulatory cells can amend T-cell behavior. Together, these changes can enhance susceptibility to infections triggered by Candida spp and dermatophytes.
For individuals exhibiting poor prognostic factors, the benefits of combination therapy might surpass the associated risks and costs, particularly for those who have not responded well to prior treatments. It is noteworthy that in numerous combination trials, individuals with poor prognostic factors or a heightened risk of recurrence were often excluded. Among studies focused solely on medication, six did not offer details on exclusion criteria, while 66.7% (6 out of 9) of studies incorporating exclusion criteria considered factors indicative of a poor prognosis or a high risk of recurrence.
In 20% (3 out of 15) of studies, there was a specified upper age limit for participants to be included in the study. In studies involving both procedures and medications, all of them provided details on exclusion criteria, and 66.7% (10 out of 15) included factors indicating a poor prognosis or a high risk of recurrence. The inclusion of upper age limits was noted in 26.7% (4 out of 15) of studies. Trials focusing on medication-based monotherapy illustrated effective outcomes in specific subgroups, including older adults, individuals battling diabetes, and those suffering from severe OM or dermatophytoma.
Hence, the combination trials published so far have excluded numerous patients who might have derived significant benefits from such therapy, including older individuals and those having compromised immune systems. Subsequent trials must focus on these patient populations to assess the efficacy of combination therapy in complex cases of OM. Given the restricted number of studies available for assessment, conflicting outcomes in studies focused solely on medication, limited effectiveness of procedural combination treatments, and the cost factor, reserving combination therapy as a secondary therapeutic option is advocated for those with stubborn cases of OM or unfavorable prognostic indicators.
The data collected indicated that combinations of oral griseofulvin with topical tioconazole or bifonazole, and oral terbinafine with topical amorolfine, showed remarkable improvements in the combination group compared to the monotherapy group across all trials. The US FDA has granted approval for the treatment of OM with oral terbinafine, itraconazole, and griseofulvin. However, griseofulvin is now less commonly used due to its inferior efficacy when compared to other treatment options, longer treatment courses, and a higher risk of adverse effects.
The US FDA has given authorization for the treatment of toenail OM with topical efinaconazole 10% and tavaborole 5% solutions, while ciclopirox 8% nail lacquer is approved for both fingernail and toenail OM. Conversely, topical tioconazole, bifonazole, and amorolfine are currently unavailable in the US. A group of expert dermatologists, podiatrists, and a microbiologist has suggested that, in the treatment of OM, efinaconazole 10% solution and terbinafine should be given priority as the primary options for topical and oral use, respectively.
Considering the recommendations, the findings in the study, and the availability of antifungal treatments in the US, oral terbinafine is suggested as the initial choice. It could be employed as a standalone therapy for uncomplicated moderate to severe cases of OM. In instances where individuals have not shown improvement with prior treatments or present unfavorable prognostic factors, the recommendation is to combine oral terbinafine with efinaconazole 10% solution, tavaborole 5% solution, or ciclopirox 8% nail lacquer. However, the selection of medication must be personalized for each patient, taking into account factors like the extent of nail involvement, the infecting pathogen, comorbidities, concurrent medications, cost, and patient preferences.
Additionally, retinoids + antifungals might be efficient in treating OM. Retinoids exhibit antimicrobial activity against fungi in both living organisms (in vivo) and laboratory settings (in vitro). Among them, tretinoin and isotretinoin exhibit the highest efficacy against M. furfur, while tazarotene is effective against dermatophytes. Additionally, tretinoin shows effectiveness against A. fumigatus and C. albicans. All-trans retinoic acid, by inhibiting the germination of hyphae necessary for biofilm formation, may also prove useful against Candida biofilm-linked infections.
These infections are frequently cumbersome to address owing to their resistance to various medications.
In a study conducted following a systematic search, 135 subjects suffering from OM affecting toenails and/or fingernails underwent a three-month treatment course with either oral itraconazole pulse monotherapy, oral acitretin monotherapy, or a combination of pulsed itraconazole and acitretin. The results portrayed mycological cure rates of 51.1%, 28.9%, and 80%, respectively. Complete cure rates were 20%, 28.9%, and 53.3% for each treatment group. Notably, the combination group exhibited a substantial improvement in OSI scores compared to both the itraconazole monotherapy and acitretin monotherapy groups. Nevertheless, there was no notable distinction witnessed between the two monotherapy cohorts.
As a result, combining retinoids with antifungals may enhance efficacy compared to using only one type of treatment. However, the validity of these findings requires confirmation through large-scale RCTs. Future research endeavors must prioritize the execution of comprehensive combination clinical trials that are both large and multicenter, employing randomization and incorporating sufficient follow-up periods. It is crucial to encompass diverse patient populations from various geographic locations to ascertain the effectiveness of combination therapy for OM treatment and to establish standardized treatment regimens. Until further studies confirm a definitive clinical advantage of combination therapy, it is suggested to consider it as a secondary choice for those facing challenging cases or exhibiting unfavorable prognostic factors.
Reserving combination therapy for OM as a second-line therapeutic option is advisable, particularly for patients exhibiting poor prognostic factors or those who do not respond satisfactorily to antifungal monotherapy, given the uncertainties surrounding its efficacy, costs, and safety considerations.
Journal of Fungi
Combination Therapy Should Be Reserved as Second-Line Treatment of Onychomycosis: A Systematic Review of Onychomycosis Clinical Trials
Julianne M. Falotico et al.
Comments (0)