Categories
Change Password!
Reset Password!


A post hoc analysis was conducted to investigate item-level alterations in the Migraine Disability Assessment (MIDAS) questionnaire in chronic migraine patients treated with Eptinezumab over a period of two years.
In people suffering from chronic migraine, sustained reduction in MIDAS-measured disability, frequency of headache, and severity of pain were observed after long-term treatment with intravenous Eptinezumab.
A post hoc analysis was conducted to investigate item-level alterations in the Migraine Disability Assessment (MIDAS) questionnaire in chronic migraine patients treated with Eptinezumab over a period of two years.
In the phase 3 PREVAIL trial, a total of 128 adults were administered eight doses of 300 mg intravenous Eptinezumab over half an hour once every 12 weeks. MIDAS was given at the baseline, Week 12, and then after every 12 weeks. Two additional MIDAS items that were not incorporated in the calculation of the total score evaluated the average headache pain intensity (from [none] 0 to [worst] 10) and number of headache days during the previous three months (MIDAS headache).
Total MIDAS scores were summed by 5 items, all measuring number of days with migraine-related disabilities in the last three months. Absenteeism was evaluated by items 5, 3 and 1, namely leisure/social/family activities (Q5), household work (Q3), and days the patient did not attend school/work (Q1). Presenteeism was measured by items 4 and 2 namely household work (Q4) or days with reduced productivity in school/work (Q2).
When compared to baseline, the mean MIDAS headache days, average headache pain severity score, mean MIDAS scores measuring absenteeism (Q1, Q3, Q5), and mean MIDAS scores measuring presenteeism (Q2, Q4) were reduced at Week 12 and Week 104, as depicted in Table 1:
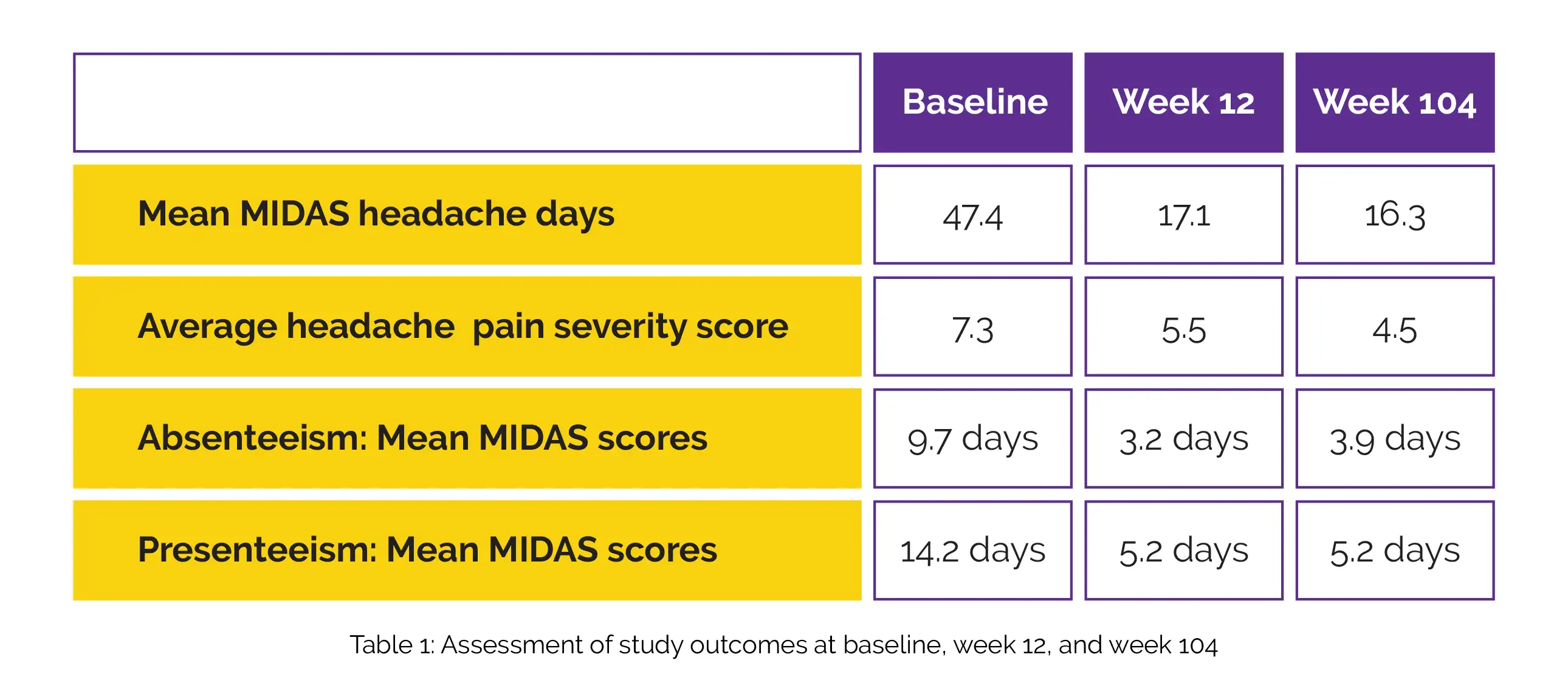
The mean total MIDAS score for patients who were classified as having a very severe MIDAS disability was 84.8, with an average decline of 56.7 days (Week 12), which was maintained at 32 days at Week 104.
Long-term use of the monoclonal antibody Eptinezumab elicited a significant and long-lasting decline in migraine-related impairment, as measured by MIDAS, consistent with the long-term decrease in headache day frequency and pain intensity.
BMC Neurology
Long-term reductions in disease impact in patients with chronic migraine following preventive treatment with eptinezumab
Andrew Blumenfeld et al.
Comments (0)