Categories
Change Password!
Reset Password!


A randomized, open-label, parallel-group phase III study (UCON trial) aimed to assess and compare the efficacy of Levonorgestrel-releasing intrauterine system (IUS) and Ulipristal acetate in lowering the impact of heavy menstrual bleeding, regardless of fibroids presence.
In females with heavy menstrual bleeding, Ulipristal is more effective in inducing amenorrhea, while both treatments, Ulipristal acetate and the Levonorgestrel-releasing intrauterine system, improve the quality of life.
A randomized, open-label, parallel-group phase III study (UCON trial) aimed to assess and compare the efficacy of Levonorgestrel-releasing intrauterine system (IUS) and Ulipristal acetate in lowering the impact of heavy menstrual bleeding, regardless of fibroids presence.
Women aged 18 and older experiencing heavy menstrual bleeding were recruited. They were randomly assigned in a 1:1 ratio to get either three 12-week therapy cycles of 5 mg Ulipristal acetate daily, with 4-week therapy-free intervals in between, or a Levonorgestrel-releasing IUS. The major endpoint, analyzed based on the intention-to-treat principle, focused on measuring the quality of life using the Menorrhagia Multi-Attribute Scale at the 12-month mark. Secondary endpoints included assessments of liver function and menstrual bleeding.
Overall, 236 females participated in the study, with recruitment temporarily halted because of concerns about the hepatoxicity of Ulipristal acetate. The subsequent discontinuation of Ulipristal acetate caused early recruitment cessation, but the trial continued with follow-up.
The major endpoint showed significant betterment in the Ulipristal group and the Levonorgestrel-releasing IUS group with an adjusted odds ratio of 0.55. With an adjusted odds ratio of 7.12, the rates of amenorrhea at 12 months were higher among those who received Ulipristal acetate compared to those who received Levonorgestrel-releasing IUS (Table 1).
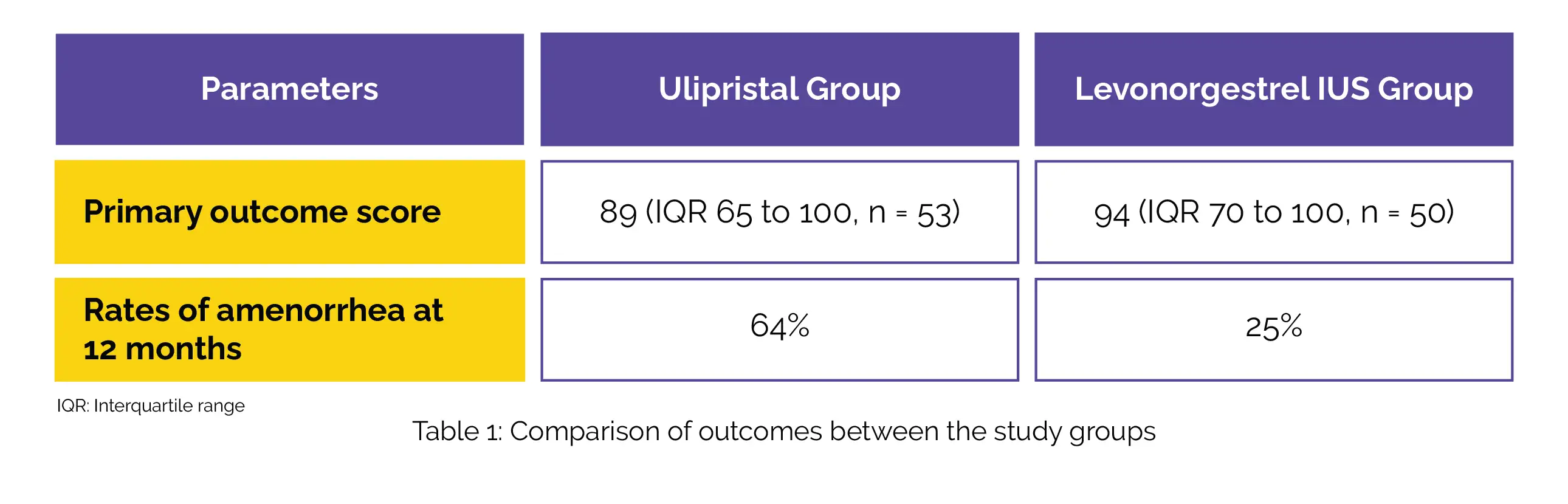
Other endpoints were comparable between the groups. No instances of endometrial malignancy or hepatotoxicity related to the usage of Ulipristal acetate were reported.
Both interventions resulted in a better quality of life. Ulipristal exhibited greater efficacy in inducing amenorrhea, showcasing its potential as an effective medical treatment option. However, it is important to note that the current utilization of Ulipristal is subject to restrictions and necessitates regular liver function monitoring.
eClinicalMedicine
Ulipristal acetate versus Levonorgestrel-releasing intrauterine system for heavy menstrual bleeding (UCON): a randomised controlled phase III trial
Lucy H.R. Whitaker et al.
Comments (0)