Categories
Change Password!
Reset Password!


A double-blind, randomized, exploratory, placebo-controlled trial aimed to assess the combined symptom and medication score (CSMS) of an optimized cumulative dosage of Pollinex Quattro (PQ) grass (27,600 standardized units [SU]), preceding the Phase III trial, under field setting.
A short course subcutaneous treatment using Pollinex Quattro grass remarkably enhances symptom and medication scores in individuals suffering from grass allergy.
A double-blind, randomized, exploratory, placebo-controlled trial aimed to assess the combined symptom and medication score (CSMS) of an optimized cumulative dosage of Pollinex Quattro (PQ) grass (27,600 standardized units [SU]), preceding the Phase III trial, under field setting.
Participants were recruited from 14 locations spanning the United States of America and Germany. A total of 119 individuals, aged 18 to 65 years, with moderate-to-severe seasonal allergic rhinitis (SAR) with or without well-managed asthma, were selected for the study. These participants received either conventional or extended PQ grass subcutaneous injections, or a placebo, encompassing a total of six pre-seasonal injections.
During peak grass pollen season (GPS), CSMS was the major effectiveness outcome of the study. Allergen-specific immunoglobulin G4 (IgG4) response and rhinoconjunctivitis quality of life questionnaire standardized (RQLQ-S) were the secondary outcomes ascertained.
In relation to the placebo group, the mean CSMS values for the standard and extended treatment plans are depicted in Table1:
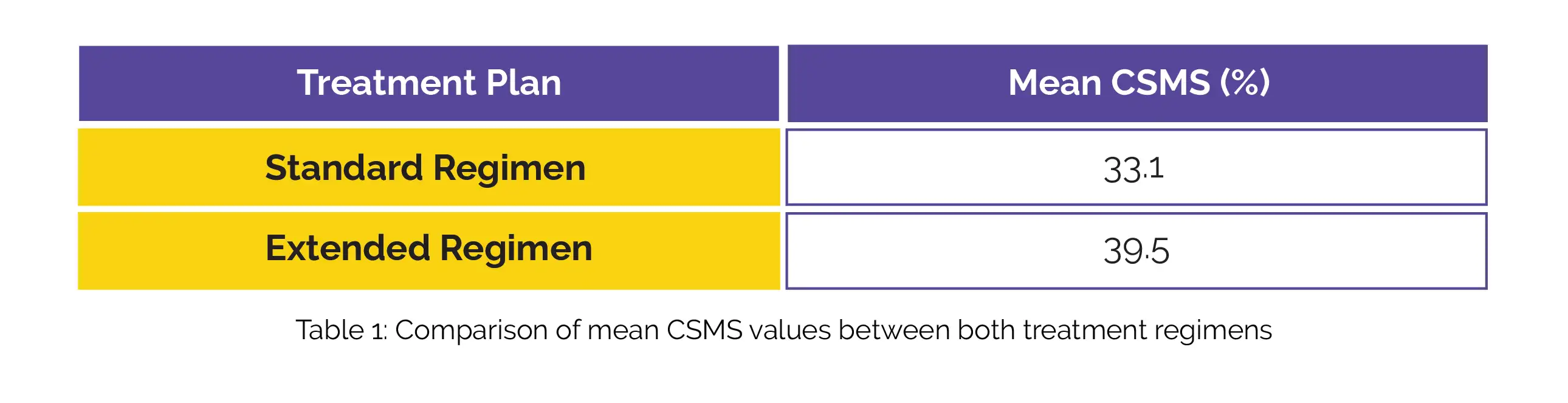
Both regimens resulted in elevated levels of IgG4, and the extended regimen exhibited a betterment in total RQLQ-S (mean change of -0.72). Moreover, both approaches had good tolerability.
This study revealed a meaningful and statistically significant effectiveness response to PQ grass. After just six injections, remarkable effect sizes of approximately/almost equal to 40% in comparison with placebo were achieved for CSMS in grass allergy patients.
Both schedules of PQ grass treatment were regarded as equally well-tolerated and safe. Due to the improved effectiveness profile, the extended regimen will advance to the pivotal Phase III trial.
Allergy
Short-course subcutaneous treatment with PQ Grass strongly improves symptom and medication scores in grass allergy
P J de Kam et al.
Comments (0)