Categories
Change Password!
Reset Password!


This parallel-group, multicentre, double-blind, randomized controlled trial was carried out to determine safety, efficacy, and tolerability of 300 mg secukinumab every 2 weeks (Q2W) vs. 300 mg secukinumab every 4 weeks (Q4W) in people having a higher body weight.
In moderate-to-severe psoriasis patients weighing 90 kg or more, secukinumab dosing every two weeks has better and sustained efficacy when compared to secukinumab dosing every four weeks.
This parallel-group, multicentre, double-blind, randomized controlled trial was carried out to determine safety, efficacy, and tolerability of 300 mg secukinumab every 2 weeks (Q2W) vs. 300 mg secukinumab every 4 weeks (Q4W) in people having a higher body weight.
The study recruited 331 obese people (weighing ≥90 kg) with chronic plaque psoriasis. Participants were randomized to get either 300 mg secukinumab Q2W or 300 mg secukinumab Q4W. Estimation of psoriasis area and severity index (PASI) 90 response at week 16 was the major endpoint ascertained. Those participants who didn't attain PASI90 at week 16 on Q4W regimen were reassigned to stay on Q4W regimen or were up-titrated to Q2W regimen.
When compared to Q4W (n=166), Q2W dosing (n=165) led to considerably greater PASI90 responses at week 16. At week 52, greater efficacy responses were maintained in the Q2W arm (n=165) when compared to Q4W (n=83), as shown in Table 1:
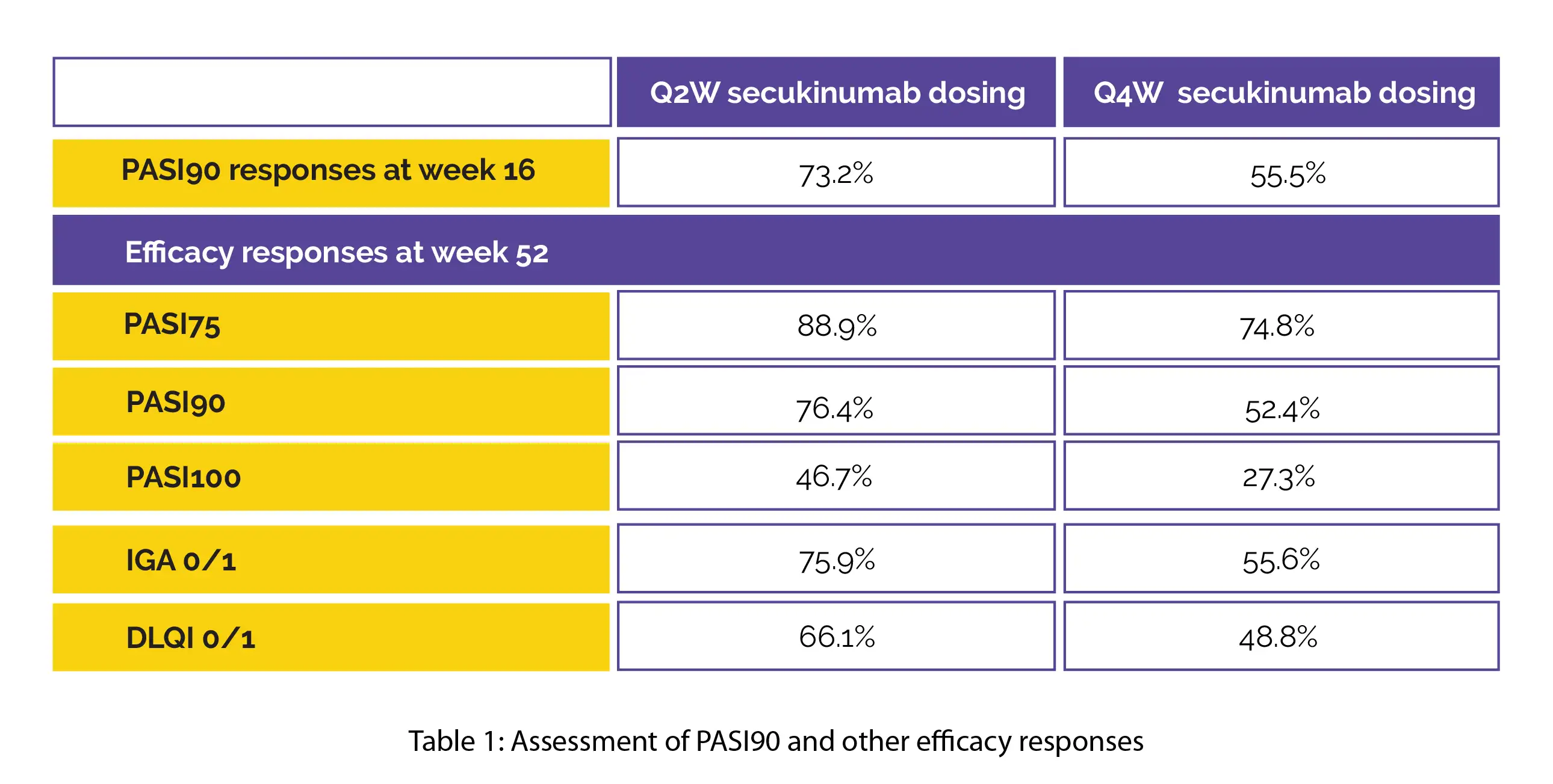
In people who did not attain PASI 90 at week 16 (PASI90 non-responders) on the Q4W regimen, up-titration to the Q2W regimen at week 16 led to improvement in the efficacy responses through week 52 after switching (PASI90: 38.7% vs. 16.5%). The safety findings were similar across the therapy arms and showed consistency with the established safety profile of this monoclonal antibody.
Over 52 weeks, 300 mg secukinumab Q2W is more effective when compared to 300 mg secukinumab Q4W in obese people suffering from psoriasis, with comparable safety results. The PASI90 non-responders derived extra benefits from up-titration to the Q2W regimen.
The British Journal of Dermatology
Secukinumab dosing every two weeks demonstrated superior efficacy compared with dosing every four weeks in patients with psoriasis weighing 90 kg or more: Results of a randomised controlled trial
Matthias Augustin et al.
Comments (0)