Categories
Change Password!
Reset Password!


The purpose of an open-label randomized control trial was to determine how shorter time duration and smaller doses of Obeticholic acid would affect subjects with nonalcoholic steatohepatitis (NASH).
Independent of diabetes and weight loss, Obeticholic acid at a lower dose of 20 mg per day for a shorter time of 24 weeks led to improvements in NASH and fibrosis.
The purpose of an open-label randomized control trial was to determine how shorter time duration and smaller doses of Obeticholic acid would affect subjects with nonalcoholic steatohepatitis (NASH).
A total of 36 non-diabetic adults with non-alcoholic fatty liver diseases (NAFLD) Activity Score (NAS) ≥ 5 were incorporated into the study. After 1:1 randomization, patients were allocated to receive Obeticholic acid 10 mg two times a day along with lifestyle modification (OCAL group) or only lifestyle modification (L group) for 24 weeks. The major endpoint was determined by NAS improvement of ≥ 2 without fibrosis worsening.
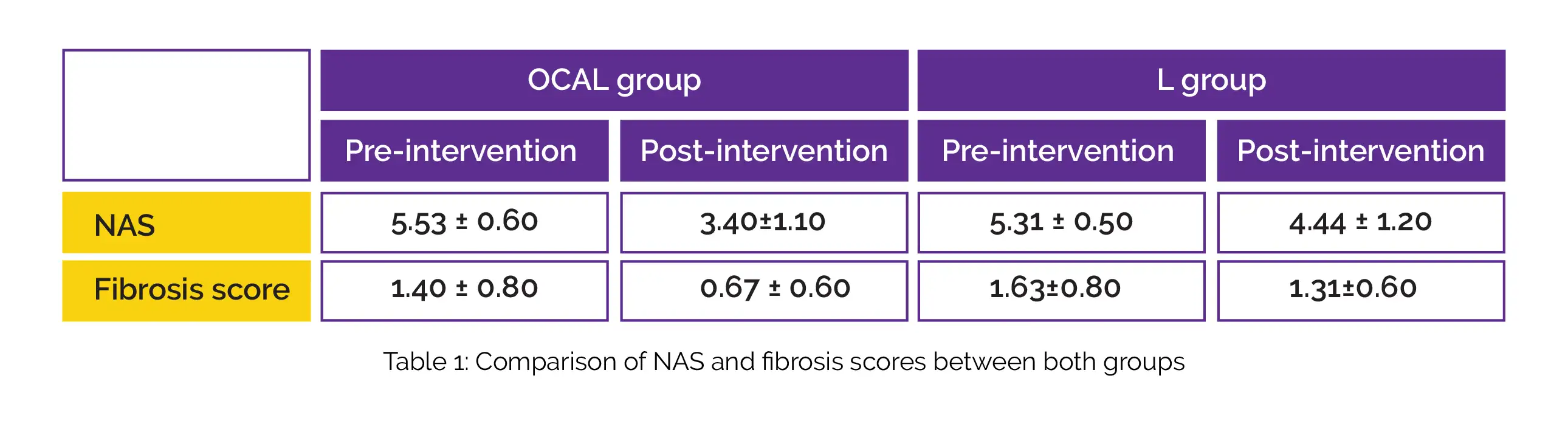
At the conclusion of the trial, 31 NASH individuals were examined according to the standard protocol. Notably, 16 of them belonged to the L group, while 15 of them were members of the OCAL group. Both groups shared the same baseline biochemical, anthropometric, metabolic, epidemiological, and histological observations. As depicted in Table 1, NAS and fibrosis scores improved after 24 weeks in the OCAL group and L group.
Overall, 68% of subjects (n = 13) in the OCAL group and 32% (n = 6) in the L group were among the respondents who experienced NASH ≥2 improvement without fibrosis deterioration. As found, 17% (n = 2) subjects in the OCAL group and 83% (n = 10) subjects in the L group made up the non-responders. The OCAL group showed a noticeable improvement in alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyl transferase (GGT). The status of diabetes and losing weight had no effect on the histological improvement.
Use of 20 mg Obeticholic acid for 24 weeks improved NAS, including its all components-fibrosis, steatosis, hepatocyte ballooning, and lobular inflammation. Both diabetic and non-diabetic NASH subjects may benefit from a shorter duration of treatment with a smaller dose of Obeticholic acid.
International Journal of Clinical and Experimental Medicine
Decreased dose and shorter duration of Obeticholic acid in nonalcoholic steatohepatitis patient: a randomized control trial
Shahinul Alam et al.
Comments (0)