Categories
Change Password!
Reset Password!


A post hoc analysis was carried out to explore if continued therapy (100 weeks) with dupilumab in an open-label extension study (LIBERTY atopic dermatitis open-label extension) enhances efficacy in people who did not attain optimal responses with short-term (16-week) monotherapy.
Continued therapy with dupilumab results in improved effectiveness in people having moderate-to-severe atopic dermatitis and not attaining optimal responses with short-term therapy.
A post hoc analysis was carried out to explore if continued therapy (100 weeks) with dupilumab in an open-label extension study (LIBERTY atopic dermatitis open-label extension) enhances efficacy in people who did not attain optimal responses with short-term (16-week) monotherapy.
It incorporated data through week 100 from three hundred and ninety-one people from dupilumab open-label extension study who did not attain at least 75% improvement from baseline in Eczema Area and Severity Index (EASI-75) or an Investigator’s Global Assessment (IGA) score of 0 or 1 with short-term (sixteen weeks, 300 mg once weekly [qw] or every 2 weeks [q2w]) dupilumab therapy in parent SOLO 1 or 2 trials. All participants were given 300 mg dupilumab qw in open-label extension study, regardless of if they were administered q2w or qw dosing in parent study.
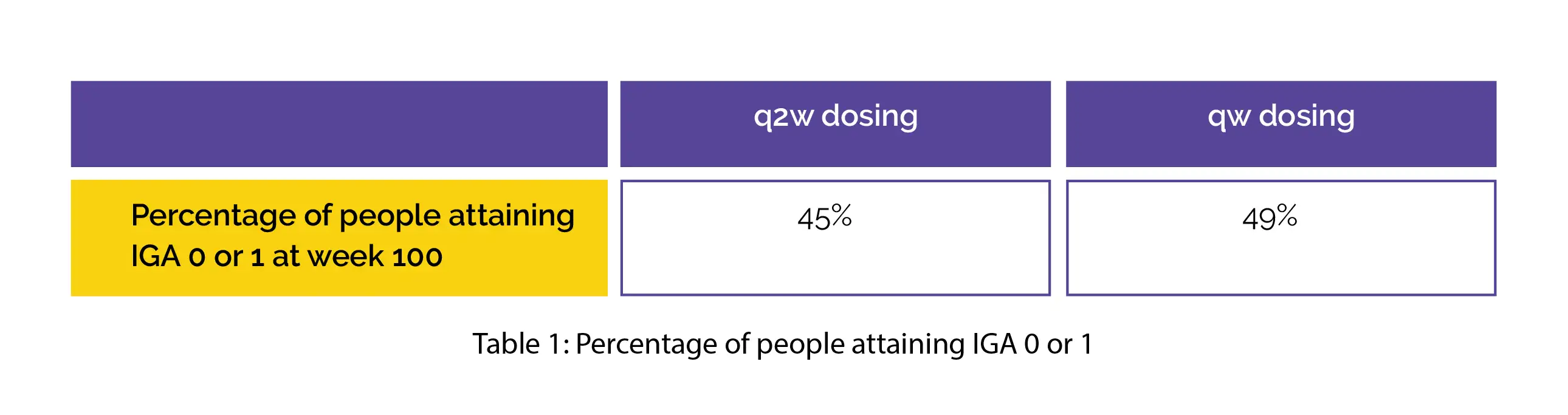
In adults who had not attained IGA 0/1 or EASI-75 during the sixteen-week parent study, the percentage of people attaining EASI-75 by week 100 was 91%. Table 1 shows the percentage of people initially on q2w and qw dosing and attaining IGA 0 or 1 at week 100.
People having suboptimal responses to short term dupilumab therapy often benefit from continued long-term therapy, irrespective of the dose frequency of the original therapy.
Long-term (100 weeks) dupilumab therapy may be linked with improvement in atopic dermatitis in individuals having suboptimal responses during the initial sixteen weeks of therapy. Irrespective of the dosing option (qw or q2w) in the parent study, most of the people were found to attain clinically meaningful benefits.
Dermatology and Therapy
Continued Treatment with Dupilumab is Associated with Improved Efficacy in Adults with Moderate-to-Severe Atopic Dermatitis Not Achieving Optimal Responses with Short-Term Treatment
April Armstrong et al.
Comments (0)