Categories
Change Password!
Reset Password!


A randomized controlled clinical trial was performed to compare the efficacy and spectrum of adverse effects of clarithromycin-based triple therapy vs. high-dose amoxicillin/bismuth regimen as a first-line eradication therapy of Helicobacter pylori (H. pylori).
Compared to standard clarithromycin-containing triple-therapy, bismuth-based high-dose amoxicillin therapy demonstrated reduced efficacy for H. pylori eradication. However, bismuth-based therapy was less frequently linked with adverse effects.
A randomized controlled clinical trial was performed to compare the efficacy and spectrum of adverse effects of clarithromycin-based triple therapy vs. high-dose amoxicillin/bismuth regimen as a first-line eradication therapy of Helicobacter pylori (H. pylori).
Overall, 579 H. pylori-positive people were divided into two groups: (I) Group 1 (standard triple therapy, n=299): Received 500 mg clarithromycin, 1000 mg amoxicillin, 40 mg esomeprazole all twice daily; (II) Group 2 (high-dose amoxicillin/bismuth-containing regimen, n=280): Received 240 mg bismuth subcitrate twice daily, 1000 mg amoxicillin three times daily, 40 mg esomeprazole twice daily. The regimens were given for fourteen days.
Collection of information on therapy completion and adverse effects was done via a telephone interview at 21-28 days following medication delivery. Using the 13C-urea breath test, H. pylori status was assessed six months after the treatment.
A total of 483 volunteers were evaluated for adverse effects (248 in Group 1 vs. 235 in Group 2) and 316 volunteers were evaluated for efficacy. Compared to Group 2, Group 1 exhibited superior efficacy in the per-protocol analysis. Regarding compliance, no inter-group difference was noted. Treatment-emergent side effects were more commonly reported in Group 1, as shown in Table 1:
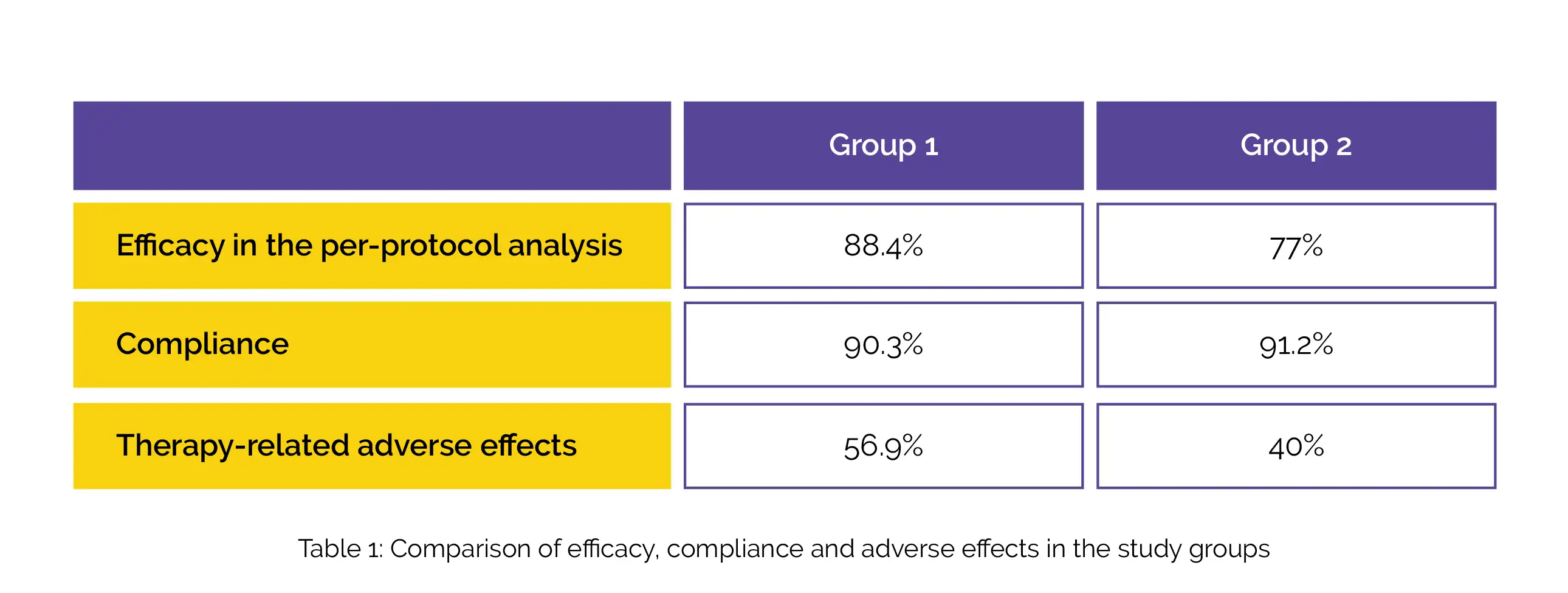
In the intention-to-treat assessment, no clinically meaningful difference in efficacy was reported.
For management of H. pylori infection, high-dose amoxicillin therapy in combination with bismuth exhibits inferior efficacy when compared to clarithromycin-based triple therapy.
European Journal of Cancer Prevention
Randomised clinical trial: Comparison of efficacy and adverse effects of a standard triple clarithromycin-containing regimen with high-dose amoxicillin and bismuth therapy in Helicobacter pylori eradication
Olga Sjomina et al.
Comments (0)