Categories
Change Password!
Reset Password!


During a 12-week double-blind period (DBP) and open-label extension (OLE), a FOCUS phase 3b research examined effectiveness of Fremanezumab in episodic or chronic migraine (EM/CM) people and prior recorded unsuitable response to two to four preventative therapy.
In episodic and chronic migraine sufferers refractory to earlier preventative drug classes, Fremanezumab exhibited long-term efficacy.
During a 12-week double-blind period (DBP) and open-label extension (OLE), a FOCUS phase 3b research examined effectiveness of Fremanezumab in episodic or chronic migraine (EM/CM) people and prior recorded unsuitable response to two to four preventative therapy.
Patients were randomly assigned (1:1:1) to receive monthly or quarterly Fremanezumab or a placebo for DBP after a baseline period of 28 days. Patients who successfully completed DBP were admitted to OLE, where everyone was treated with Fremanezumab on a monthly basis. DBP group summarized the results.
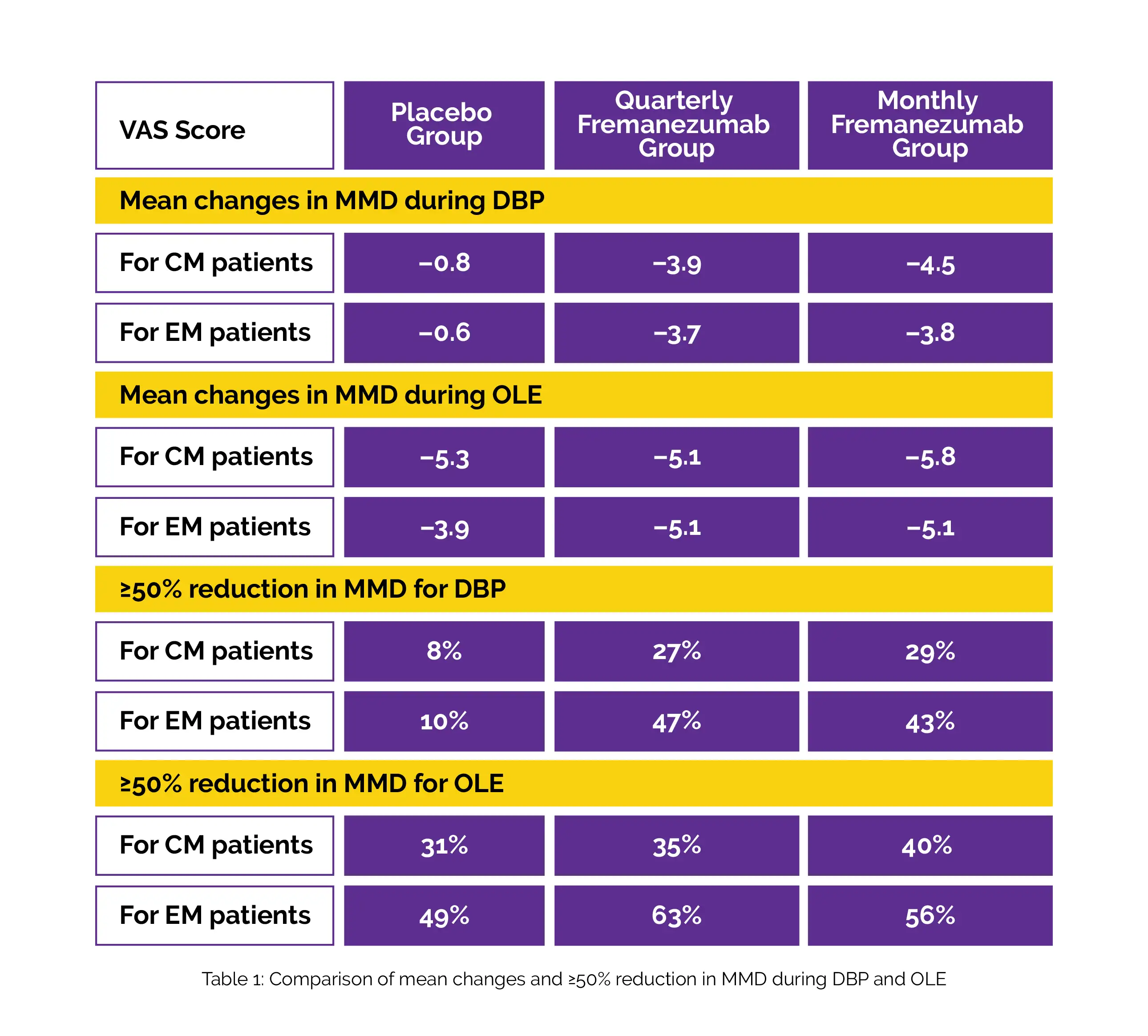
Table 1 illustrates the least squares mean changes in monthly migraine days (MMD) in placebo, quarterly, and monthly Fremanezumab groups for CM patients (n=509) and for EM patients (n=328), mean MMD changes for EM patients (n=313) and for CM patients (n=493) during OLE and DBP. For individuals with CM and EM, the monthly headache days of at least moderate severity decreased during DBP and elevated during OLE. For DBP and OLE, ≥50% reduction in MMD in placebo, quarterly, and monthly Fremanezumab groups for CM and EM individuals was observed.
The monoclonal antibody Fremanezumab showed sustained effectiveness in EM and CM people who had unsatisfactory responses to several preventative classes.
Journal of Neurology, Neurosurgery & Psychiatry
086 Long-term efficacy of Fremanezumab in migraine patients with inadequate response to prior preventive medication classes
Steffen Naegel et al.
Comments (0)