Categories
Change Password!
Reset Password!


This phase 2 randomized trial explored the safety and immunogenicity of RSVPreF3 in mothers and their infants. The RSV-particular antibody levels and neutralizing antibody (nAb) titers in babies born to vaccinated mothers were also assessed.
A single dose of maternal investigational Respiratory Syncytial Virus Vaccine (RSVPreF3) given during the late 2nd or 3rd trimester of pregnancy was well-tolerated and exhibited an acceptable safety profile with respect to pregnancy-associated or neonatal adverse events or pregnancy outcomes.
This phase 2 randomized trial explored the safety and immunogenicity of RSVPreF3 in mothers and their infants. The RSV-particular antibody levels and neutralizing antibody (nAb) titers in babies born to vaccinated mothers were also assessed.
The safety of RSVPreF3 dosed 60 µg or RSVPreF3 dosed 120 µg was assessed during the late second or third trimester in a total of 213 healthy pregnant women aged 18 to 40 years. They were examined through 6 months’ post-partum and their offspring through initial months. The immunogenicity was assessed through day 43 post-partum and day 181 after birth.
RSVPreF3 was found to be acceptable as none of the severe adverse events for pregnant women or infants were associated with the use of vaccine under consideration. The main results were apparent in the 60 µg RSVPreF3 and 120 µg RSVPreF3 groups:
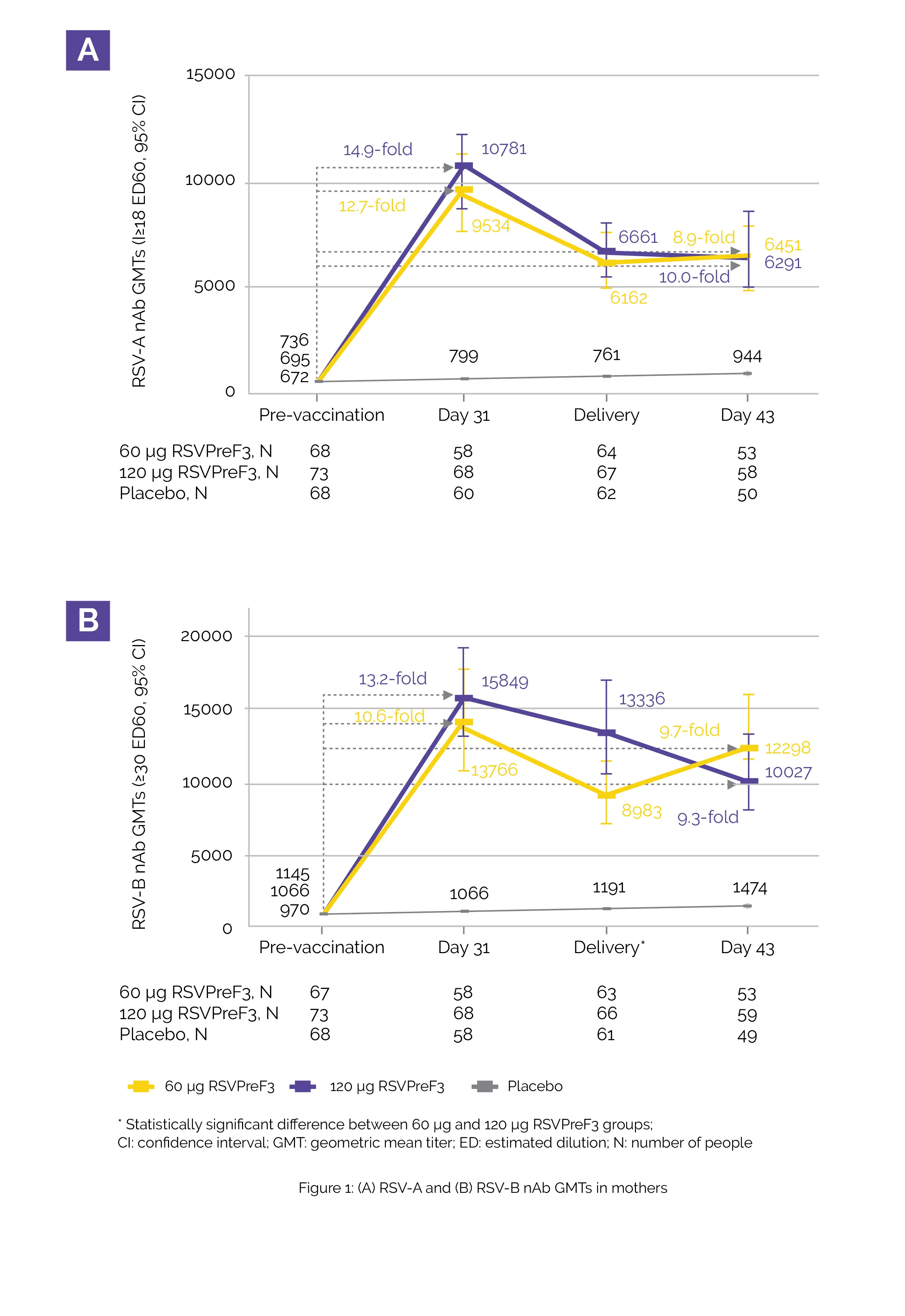
RSVPreF3 maternal vaccination was well-tolerated and had an acceptable safety risk profile. It encouraged robust RSV-specific immune responses with positive antibody transfer to the infants from vaccinated mothers.
The Journal of Infectious Diseases
Safety and Immunogenicity of an Investigational Respiratory Syncytial Virus Vaccine (RSVPreF3) in Mothers and Their Infants: A Phase 2 Randomized Trial
Zourab Bebia et al.
Comments (0)