Categories
Change Password!
Reset Password!


To assess the effectiveness and safety of Odevixibat, an inhibitor of the ileal bile acid transporter, compared to placebo, in patients with a rare multisystem disorder- Alagille syndrome (also called arteriohepatic dysplasia).
As a non-surgical option, Odevixibat has the potential to elevate the quality of care for individuals with Alagille syndrome by relieving itching and decreasing the serum bile acids.
To assess the effectiveness and safety of Odevixibat, an inhibitor of the ileal bile acid transporter, compared to placebo, in patients with a rare multisystem disorder- Alagille syndrome (also called arteriohepatic dysplasia).
The ASSERT study enrolled participants from 21 medical facilities throughout 10 countries, including Belgium, France, Germany, Italy, Malaysia, Turkey, the Netherlands, Poland, the UK, and the USA. Suitable candidates had genetically confirmed Alagille syndrome, a history of significant itching, and raised serum bile acids.
Through a randomized process (2:1), patients were assigned to receive either Odevixibat 120 μg/kg daily orally or a placebo for 24 weeks. Allocation was done through a web-based system, employing block sizes of six and stratification by age (younger than 10 years old and those who are 10 years or older but younger than 18 years old).
The primary efficacy endpoint was the alteration in the scratching score using the PRUCISION instrument (varying from 0-4) from the start to weeks 21 through 24. The alteration in serum bile acid concentration from the starting point to the average of weeks 20 and 24 was considered a pre-determined key secondary efficacy measure. The analysis included patients who received a minimum of one dose of the study drug.
Fifty-two patients (median age = 5·5 years; 52% males) were randomly divided into two groups: one receiving Odevixibat (number of patients = 35) and the other receiving a placebo (number of patients = 35). The difference in the mean scratching scores has been shown in the following table 1:
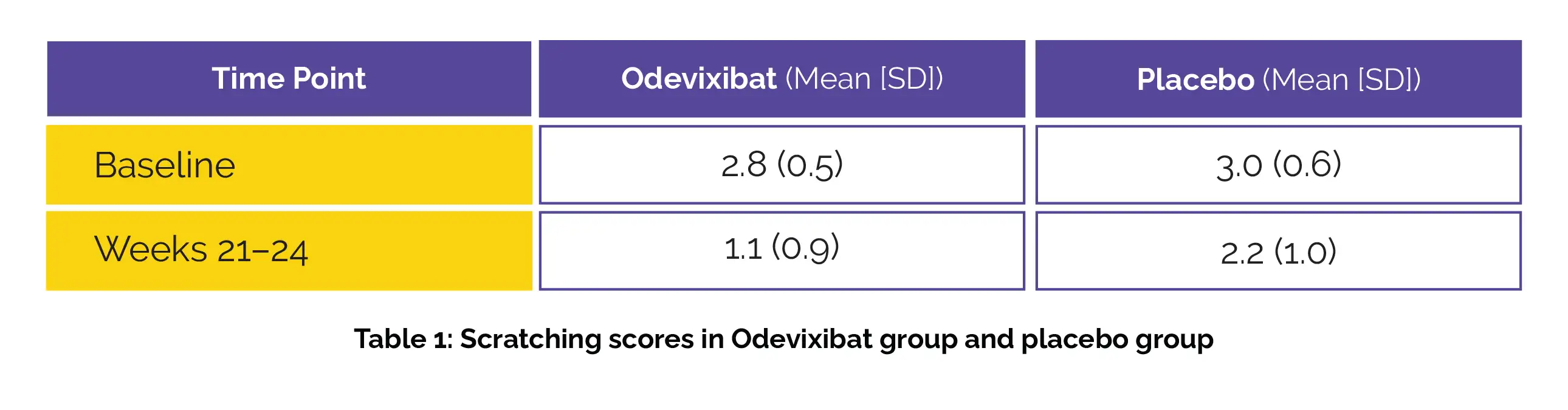
The least-squares (LS) mean change from baseline was -1.7 (95% CI -2.0 to -1.3) for Odevixibat and -0.8 (-1.3 to -0.3) for placebo (Figure 1):
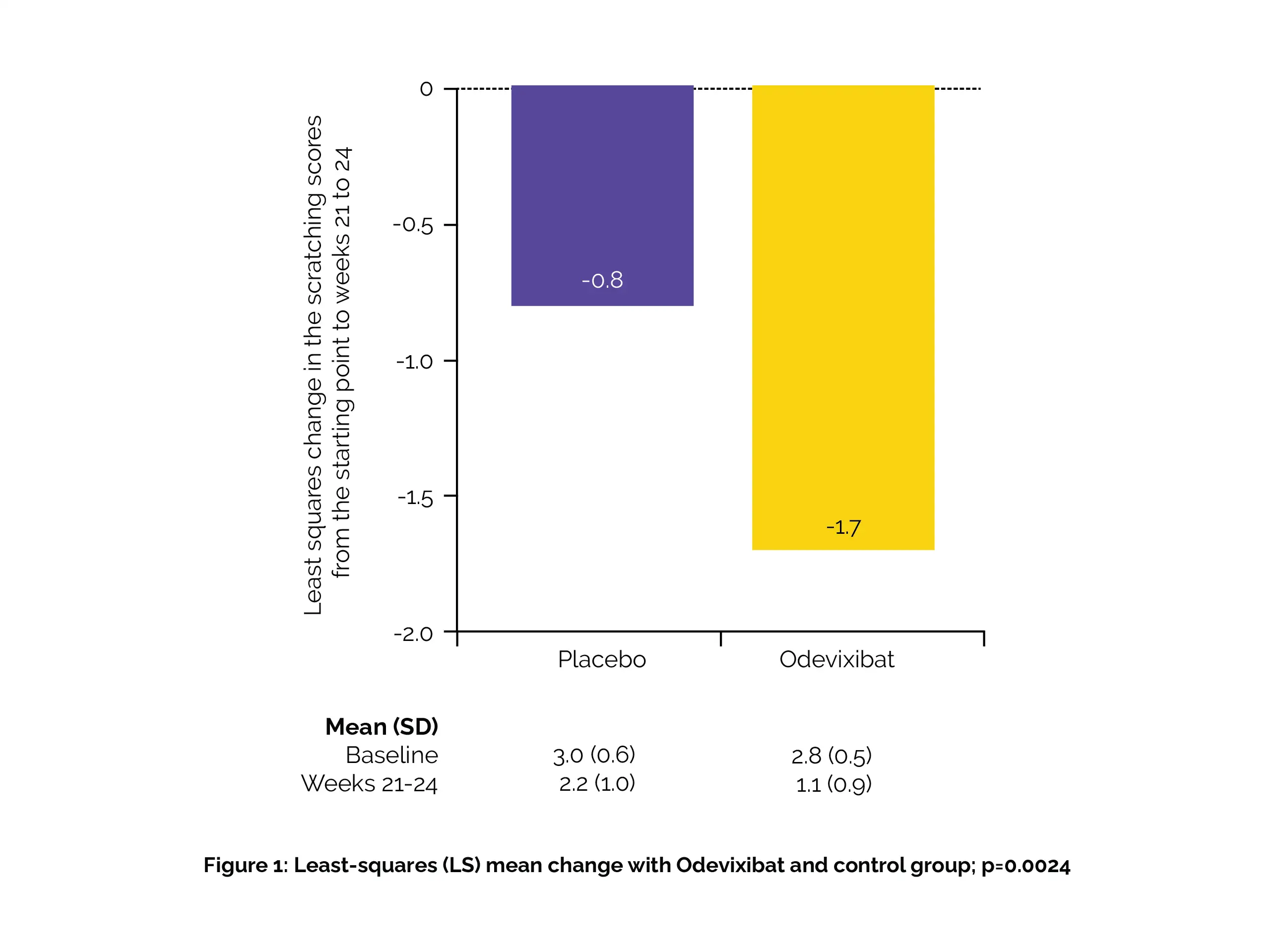
A significantly greater reduction in scratching score for Odevixibat compared to placebo (p=0.0024) was observed.
Also, Odevixibat led to significantly greater declines in mean serum bile acids from the start versus placebo (237 μmol/L with Odevixibat versus 246 μmol/L with placebo). Diarrhoea was the most frequently reported adverse event (10 patients out of 35 patients in the Odevixibat group compared to 1 patient out of 17 in the placebo group. There were also cases of pyrexia reported by 8 patients in the Odevixibat group versus 4 patients in the control group. Severe adverse events were reported by 5 individuals in the main intervention group and 2 in the placebo group. Treatment discontinuation did not occur among any of the patients, and there were no fatalities.
Odevixibat shows promise as a non-surgical approach to alleviate itching, lower serum bile acids, and enhance the quality of care for individuals with Alagille syndrome. Future insights into the extended safety and effectiveness of Odevixibat in this patient group are anticipated.
The Lancet Gastroenterology
Efficacy and safety of Odevixibat in patients with Alagille syndrome (ASSERT): a phase 3, double-blind, randomised, placebo-controlled trial
Nadia Ovchinsky et al.
Comments (0)