Categories
Change Password!
Reset Password!


Diabetic peripheral neuropathy, the most common form of neuropathy in developed countries impacts an estimated 50% of individuals suffering from diabetes and thus poses a huge economic burden.
Capsaicin, pregabalin, mirogabalin, duloxetine, lacosamide, and tapentadol showed superior efficacy when compared to placebo to control pain linked with diabetic peripheral neuropathy. On the other hand, gabapentin and ABT-894 demonstrated no profound effect.
Diabetic peripheral neuropathy, the most common form of neuropathy in developed countries impacts an estimated 50% of individuals suffering from diabetes and thus poses a huge economic burden. Symmetric, distal, and chronic sensorimotor polyneuropathy is the most common form. On the other hand, asymmetric or focal neuropathy like trunk radiculopathy, diabetic muscle atrophy, and compression palsy are the uncommon forms.
The American Academy of Neurology, and the American Association of Neuromuscular and Electrical Diagnostic Medicine have issued comprehensive reviews of therapies for diabetic peripheral neuropathy. In the year 2011, the American Academy of Physical Medicine and Rehabilitation issued an article illustrating that in people with diabetic peripheral neuropathy, pregabalin is an efficacious therapy while other therapies like amitriptyline and venlafaxine might also be effective.
RATIONALE BEHIND RESEARCH
In the year 2017, a systematic review of the latest randomized controlled trials (RCTs) of agents for diabetic peripheral neuropathy pain was issued. But, this review did not integrate some novel drugs and did not integrate evidence from people who reported a 30% or 50% pain decline.
Thus, this systematic review was carried out to demonstrate the benefits and disadvantages of drug regimens to relieve diabetic peripheral neuropathy pain and improve health-linked quality of life.
OBJECTIVE
The present systematic review was conducted to systematically determine the effects of various drugs to alleviate painful diabetic peripheral neuropathy.
Literature search
Databases like PubMed, MEDLINE, and Embase were searched (from 1st January 2008 to 1st June 2020). The Preferred Reporting Items of the Systematic Review and Meta-Analysis (PRISMA) guidelines were followed. The language was limited to English.
Inclusion criteria
A placebo-controlled, double-blind RCT investigating the influence of different analgesic agents on people (age eighteen years and more) having painful diabetic peripheral neuropathy.
Exclusion criteria
Following studies were excluded:
Study selection and Data extraction
Screening and identification of studies were done by 2 reviewers. Through discussion, the differences were settled out. Furthermore, a manual search of references in the issued systematic reviews and meta-analyses was carried out to ascertain that there were no missing studies.
Independent extraction of the data to an excel spreadsheet was done as per the predefined standards. For each of the incorporated studies, extraction of data like baseline characteristics, study time, intervention measures and time, demographics, and trial design was done.
Risk of Bias and Quality assessment
With the aid of the Cochrane Collaboration Risk Assessment tool, the risk of bias was determined for each incorporated study. For the continuous variables, standardized mean difference (SMD) and 95% confidence interval (CI) was utilized. The risk ratio of 95% CI was estimated for dichotomous variables. For investigating the efficacy of drugs and placebos, an assessment of alteration was done prior to and after the treatment. P=0.05 was considered statistically significant.
RevMan V.5.3 (meta-analysis software) was utilized for the assessment. Heterogeneity was assessed according to I2. An I2 value of 75%, 50%, and 25% were judged as substantial, moderate, and mild heterogeneity, respectively. The heterogeneity was solved by subgroup assessment. For rating the overall quality of evidence for each endpoint as per 5 evaluation criteria (indirectness, publication bias, risk of bias, inconsistency, and imprecision), GRADE Pro (V.3.6) software was utilized.
Study outcomes
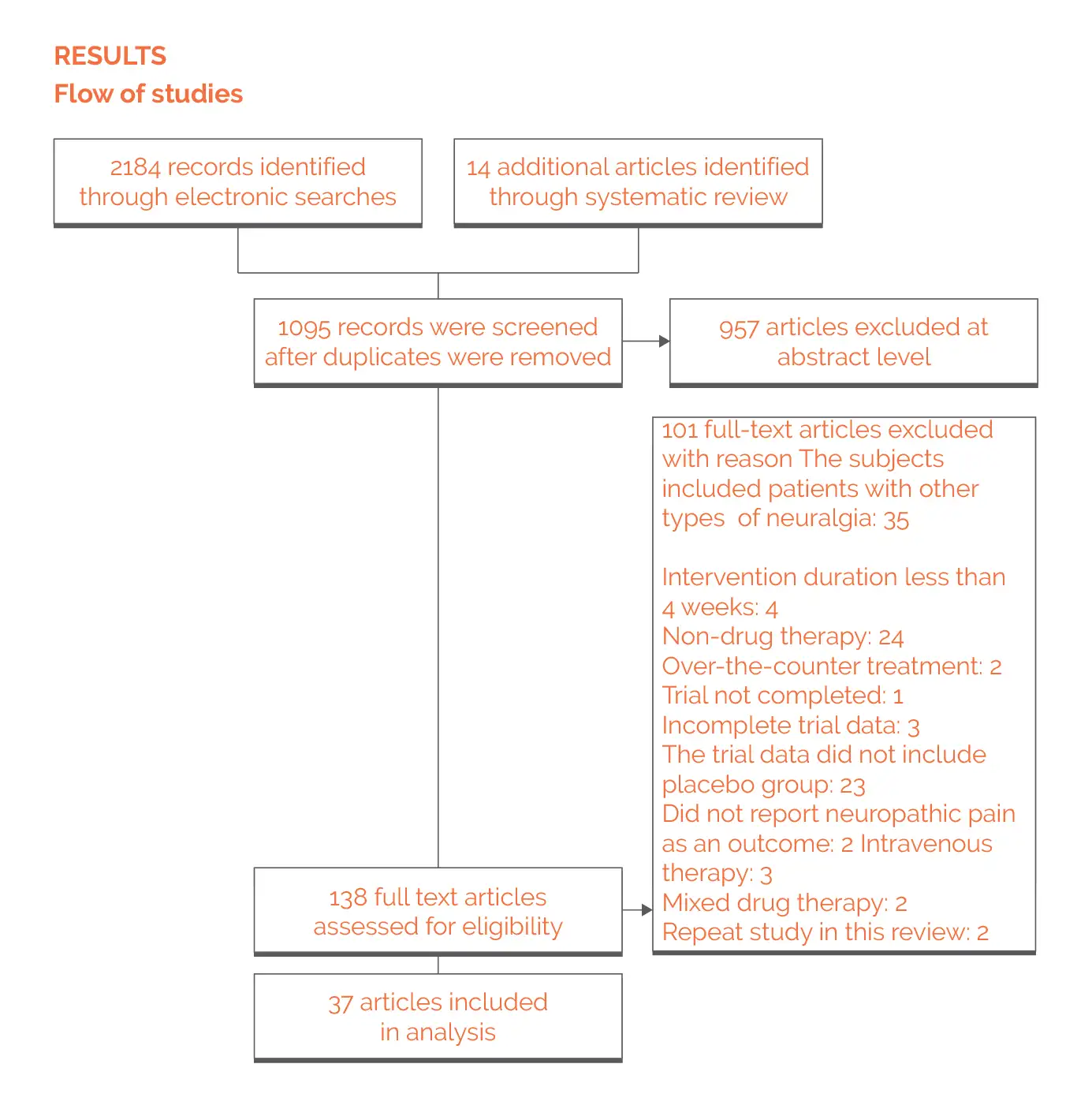
Outcomes
Study and participant characteristics:
Effect of intervention on the outcome:
On the basis of available studies issued till now, a meta-analysis was carried out to explore different drugs to combat painful diabetic peripheral neuropathy. As found, tapentadol, duloxetine, lacosamide, pregabalin, and capsaicin were all highly effective when compared to placebo. However, the quality of evidence was low. The guidelines of American Academy of Neurology and European Neurological Association suggest pregabalin as the first-line clinical drug.
This study demonstrated profound differences in pain scores and 30% and 50% pain decline in pregabalin-treated people when compared to placebo-treated people. High heterogeneity of pregabalin was noted owing to the large number of incorporated studies and the inconsistency of research methodology and pain test standards in various articles. Thus, a subgroup assessment was utilized for reducing its heterogeneity. The findings of the subgroup assessment revealed higher efficacy of pregabalin when compared to placebo.
Furthermore, the study demonstrated that few people do not tolerate elevated doses of pregabalin well. In such cases, the effect of the lower dose may not be sufficient, although dose escalation may be precluded by noxious reactions. Furthermore, ethnic factors can also play a pivotal role. Despite the fact that pregabalin and gabapentin have a comparable mechanism of action, the two agents are often utilized interchangeably in clinical management. However, the findings of the study illustrated that gabapentin has no comparable effect when compared to placebo.
ABT894, the newly developed drug, also illustrated no notable difference in comparison with placebo. The pain-relieving effects of gabapentin and ABT-894 need to be further investigated with longer and larger RCTs. Regarding pain scores, even though lacosamide and capsaicin illustrated profound differences when compared to placebo, there was no substantial difference in their 30% pain decline when compared to placebo.
Though lacosamide and capsaicin can minimize pain in people with painful diabetic peripheral neuropathy, however, they might not provide a considerable degree of pain reduction. Moreover, unlike lacosamide, the risk of noxious reactions is considerably elevated with capsaicin. Mirogabalin was developed particularly to mitigate peripheral neuropathic pain. As found, mirogabalin could remarkably decrease the pain score. Regarding 50% pain decrease, no notable difference was noted when compared to placebo.
Duloxetine (selective serotonin and norepinephrine reuptake inhibitor) has been extensively utilized in medical care practice. Considering the side effects, capsaicin, pregabalin, duloxetine, and tapentadol exhibited a higher risk of adverse events when compared to placebo. ABT894, lacosamide, and gabapentin exhibited no profound difference. Furthermore, the evidence demonstrated that the risk of noxious events associated with tapentadol and capsaicin is considerably greater when compared to duloxetine and pregabalin.
According to the studies, pregabalin's tolerability is comparable or slightly worse when compared to placebo. Investigators explored the safety data of western RCTs assessing pregabalin for painful diabetic peripheral neuropathy with data from 2 similar trials of East Asian origin. It was noted that people of East Asian origin exhibit more common adverse effects like weight gain, dizziness, peripheral edema, and drowsiness when compared to whites.
The relatively low average weight of people of East Asian origin and greater exposure to pregabalin might be the reason for this. This, in turn, results in decreased tolerance and a considerable drop in the average prescribed dose. Since the majority of trials have been performed in Europe/North America, race does not appear to exert a substantial impact on pregabalin's pharmacokinetics. There is a requisition for more high-quality robust trials in East Asia for further verifying the ethnic differences in pharmacodynamics.
Regarding 30% and 50% pain decline, this study revealed the superiority of duloxetine over pregabalin. Furthermore, profound differences were noted in terms of adverse events. Thus, duloxetine might have a better impact on painful diabetic peripheral neuropathy. The results were compared with other studies assessing different drug therapies for painful diabetic peripheral neuropathy. The findings were somewhat consistent when compared to other studies.
In 2008, a meta-analysis on pregabalin's influence on painful diabetic peripheral neuropathy patients was carried out. Regarding pain linked with painful diabetic peripheral neuropathy, pregabalin revealed higher efficacy when compared to placebo. Zhang et al. illustrated that pregabalin was highly effective when compared to placebo for management of diabetic peripheral neuropathy-linked pain. However, they did not base their results on the baseline alterations between groups.
The effect of pregabalin on neuralgia was recently investigated. Pregabalin exhibited a good impact on individuals suffering from peripheral neuralgia. However, this study incorporated individuals suffering from postherpetic neuralgia. Furthermore, these studies only assessed pregabalin's effectiveness. In a study by Waldfogel et al., both tapentadol and pregabalin exhibited remarkable therapeutic effects on painful diabetic peripheral neuropathy.
But, 0.075% capsaicin displayed no profound difference when compared to placebo, primarily due to the comprehensive assessment of capsaicin at distinct concentrations. For most of the studies, the intervention duration was less than three months. Only a few studies had an intervention duration greater than three months. Since painful diabetic peripheral neuropathy itself needs long-term therapy, these agents are commonly utilized in the clinic as long-term drugs for improving symptoms in people suffering from painful diabetic peripheral neuropathy.
Thus, the long-term impact or noxious reactions of these medicines could not be assessed. For opioids, tapentadol was noted to have a better therapeutic effect on painful diabetic peripheral neuropathy. The study revealed that tapentadol exhibited better pain scores and 30% and 50% pain alleviation rates when compared to other medicines. Tapentadol exhibited the highest drop in pain scores in comparison with the placebo group. But, since all the included studies were short-term studies, the findings demonstrated that tapentadol considerably raised the risk of noxious events.
Due to the paucity of evidence of long-term efficacy and growing evidence of the severe risks of opioids (mainly abuse and addiction), the guidelines of American Academy of Neurology does not suggest opioids to treat chronic pain. Large sample RCTs with long-term follow-up periods and strict criteria must be designed for further authenticating the efficacy and safety of these agents. This will assist in better guidance of clinical decision-making.
For management of diabetic peripheral neuropathy pain, tapentadol, duloxetine, and pregabalin exhibited good efficacy. These FDA-approved agents are most commonly used for clinical management of diabetic neuropathy. Capsaicin, lacosamide, and mirogabalin also showed better effect than placebo while ABT-894 and gabapentin did not show any substantial effect when compared to placebo.
Frontiers in Neurology
Different Drugs for the Treatment of Painful Diabetic Peripheral Neuropathy: A Meta-Analysis
Lian Jingxuan et al.
Comments (0)