Categories
Change Password!
Reset Password!


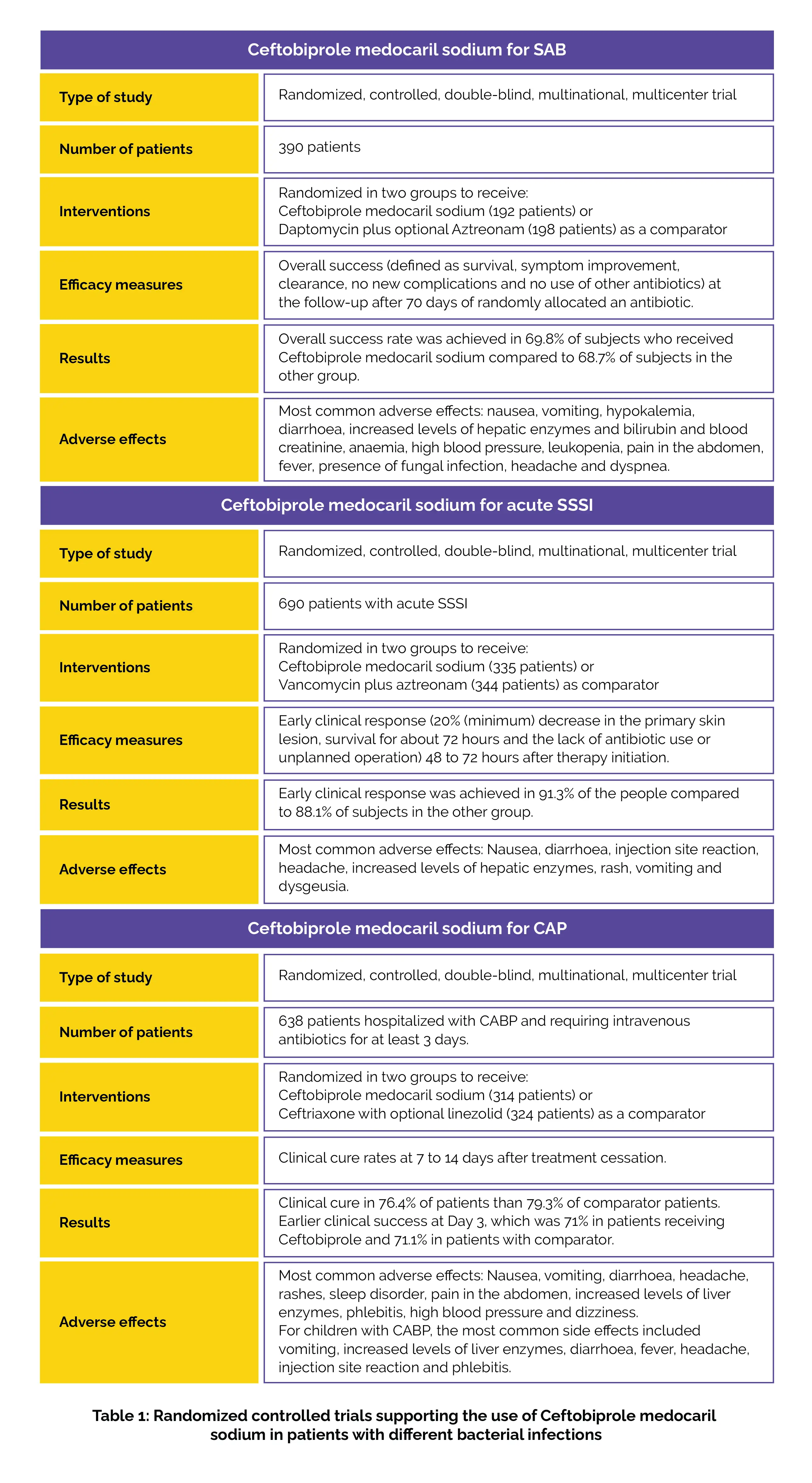
Ceftobiprole medocaril sodium injection is effective for 3 acute bacterial infections.
FDA’s press release in April 2024 announced its approval of ceftobiprole medocaril injection treatment for adults with Staphylococcus aureus bacteremia (SAB), right-sided native valve infective endocarditis, acute skin and skin structure infections (SSSI). And community-acquired pneumonia (CAP) in children (aged 3 months-<18 years) and adults.
This approval is based on the following trials comprising ceftobiprole medocaril sodium injection for different bacterial infections:
Important considerations before starting Ceftobiprole medocaril sodium:
Patients should avoid using Ceftobiprole medocaril sodium if they have a documented history of severe hypersensitivity to ceftobiprole, any of its components, or other cephalosporin antibiotics.
Ceftobiprole carries specific cautions and advisories, including a potential increase in mortality among patients with ventilator-associated bacterial pneumonia (an off-label use), as well as risks of hypersensitivity reactions, seizures, other central nervous system effects and diarrhoea due to Clostridioides difficile.
FDA News Release
https://www.fda.gov/news-events/press-announcements/fda-approves-new-antibiotic-three-different-uses
FDA Approves New Antibiotic for Three Different Uses
Comments (0)