Categories
Change Password!
Reset Password!


In GERD patients, 40 mg Esomeprazole and 20 or 40 mg Vonoprazan have comparable effectiveness and safety.
For gastroesophageal reflux disease (GERD) management, Vonoprazan and Esomeprazole were shown to have equivalent safety and effectiveness in a randomized phase 2 clinical study. Jan Tack et al. compared the effectiveness of potassium-competitive acid blocker versus proton-pump inhibitor (PPI) in preventing heartburn symptoms in GERD patients who had a partial response to Esomeprazole throughout the course of a 4-week therapy period.
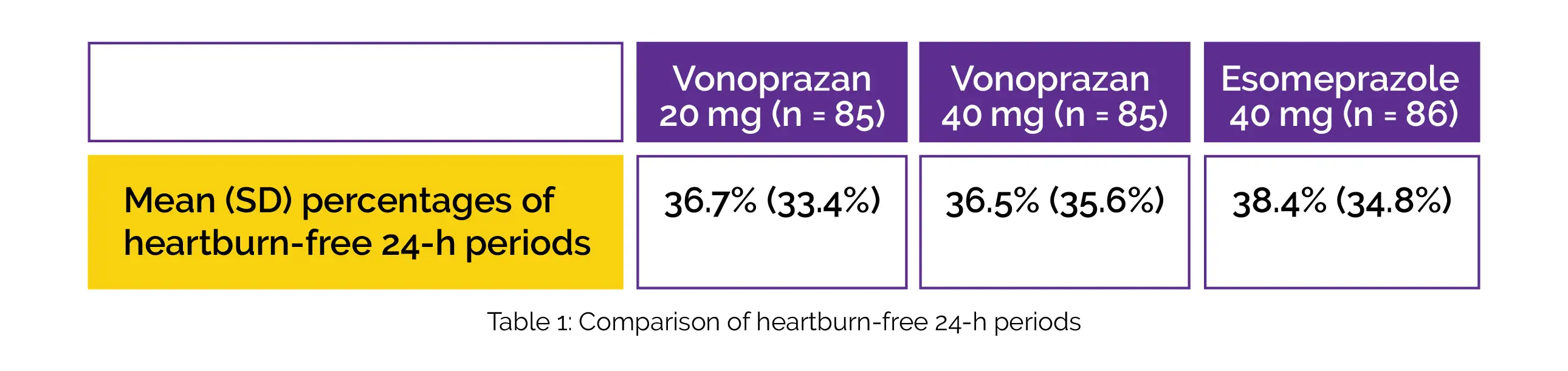
Overall, 256 patients (mean age 52.6 years; females, 59.4%) who had GERD symptoms and a partial response to a healing dosage of Esomeprazole were randomly assigned to receive Vonoprazan 20 mg once a day (q.d.) (n = 85) and 40 mg (n = 85) vs. Esomeprazole 40 mg q.d (n = 86). Regarding the mean (standard deviation [SD]) percentage of heartburn-free 24-hour periods, there was no intergroup statistical significance, as depicted in Table 1:
From a dose of 20 mg to a dose of 40 mg, Vonoprazan exposure rose proportionately (mean Cmax: 23.3 ng/ml to 47.1 ng/ml, respectively). No fatalities were witnessed, and the majority of treatment-emergent adverse events were reported to be mild. Vonoprazan was well-tolerated, and there was no clinically meaningful difference across treatment groups in terms of safety and effectiveness.
Neurogastroenterology & Motility
Randomized clinical trial: A double-blind, proof-of-concept, phase 2 study evaluating the efficacy and safety of Vonoprazan 20 or 40 mg versus Esomeprazole 40 mg in patients with symptomatic gastro-esophageal reflux disease and partial response to a healing dose of a proton-pump inhibitor
Jan Tack et al.
Comments (0)