Categories
Change Password!
Reset Password!


In migraine patients, tiapride effectively and safely reduces migraine days per month when compared to topiramate.
A randomized, double-blind, pilot study depicted that compared to topiramate, tiapride is a safe, efficient and well-tolerated prophylactic intervention for people diagnosed with chronic migraine. Researchers sought to assess safety and effectiveness of tiapride vs. topiramate for chronic migraine prophylaxis. Overall, 56 people (18-65 years of age) suffering from chronic migraine were recruited and segregated into two intervention groups. For 12 weeks, patients in group 1 were given 100 mg tiapride two times a day and patients in group 2 received 25 mg topiramate two times a day.
Alteration in the monthly average number of migraine days was the major outcome ascertained. Measurements were carried out to explore alteration in headache effect as estimated by Headache Impact Test-6, alteration in monthly number of headache days, and percentage of patients with more than 50% and 75% reduction in their monthly migraine days. Overall, 39 subjects (tiapride = 21; topiramate = 18) were incorporated in the intention-to-treat population.
Out of 39, 35 subjects (tiapride = 18; topiramate = 16) finished the study. A mean reduction of migraine days per month in the tiapride group and topiramate group is shown in Table 1:
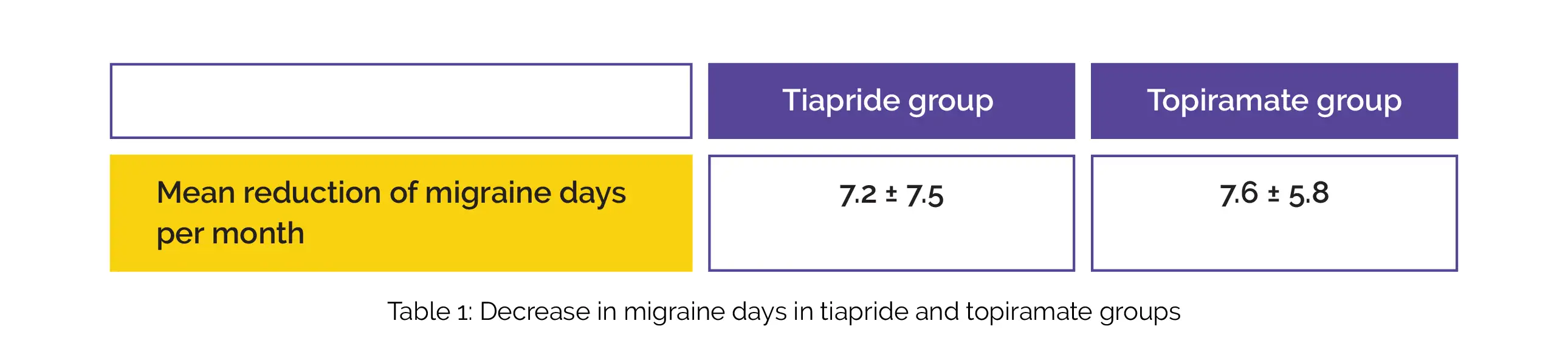
Regarding other effectiveness variables measured, no inter-group differences were noted. Mild adverse effects were witnessed in both groups. Hence, tiapride was associated with promising prophylactic effects in individuals with chronic migraine when compared to topiramate.
Revue Neurologique
[Comparison of tiapride and topiramate in the prophylactic treatment of chronic migraine: a randomised, double-blind, pilot study]
S Juica-Avello et al.
Comments (0)