Categories
Change Password!
Reset Password!


Topical tapinarof was effective for the treatment of patients with mild-to-severe plaque psoriasis.
As per the findings of a recent phase 3 randomized trial, tapinarof 1% cream was found superior for reducing the severity of psoriasis when administered for 12 weeks. Researchers undertook this study to determine safety and efficacy of tapinarof cream for treating psoriasis. Patients with a baseline Physician’s Global Assessment (PGA) score of 2 (mild) to 4 (severe) and 3 to 20% of total body surface affected were enrolled.
Participants were given either tapinarof 1% cream or vehicle cream once daily for twelve weeks. The primary outcomes included assessment of PGA score and a decrease of at least 2 points from baseline at 2 weeks. The secondary outcomes were the mean change in percent of body-surface area affected from baseline, PGA score of 0 or 1, at least a 75% reduction and 90% reduction in the Psoriasis Area and Severity Index (PASI) score.
Mean alteration in the percentage of people with a reduction of at least four points in Psoriasis Symptom Diary score, Peak Pruritus Numeric Rating Scale (PP-NRS) score, Dermatology Life Quality Index total score, and PP-NRS total score from baseline to 12 weeks were patient-reported outcomes (PROs).
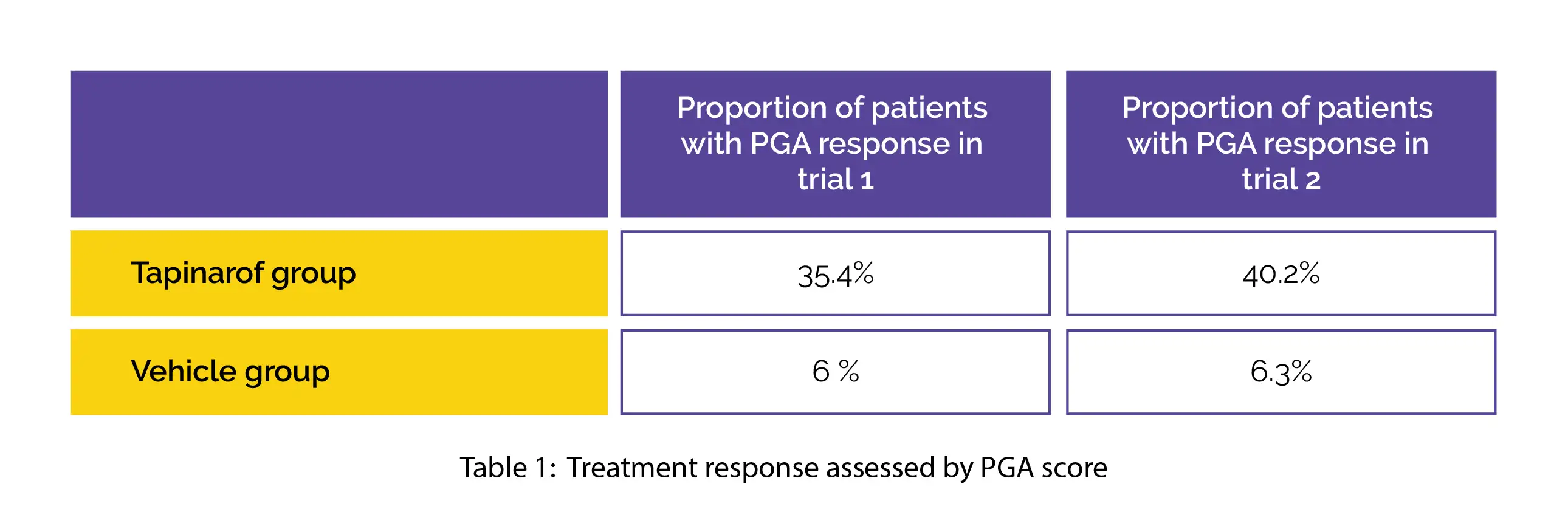
A total of 692 patients were screened in trial 1, among which 510 were enrolled. Similarly, 674 were screened in trial 2, among which 515 patients were included. Treatment response evaluated by PGA score was observed as below:
The findings of secondary outcomes and PROs were found to be similar to the primary endpoints. However, tapinarof cream was associated with various adverse events such as nasopharyngitis, folliculitis, upper respiratory tract infection, pruritus, contact dermatitis and headache.
From the study findings, it can be concluded that tapinarof 1% cream can be used for the treatment of psoriasis but there is a need to conduct larger trials to determine and compare the efficacy and safety of tapinarof cream to other psoriasis treatments.
The New England Journal of Medicine
Phase 3 Trials of Tapinarof Cream for Plaque Psoriasis
Mark G. Lebwohl et al.
Comments (0)