Categories
Change Password!
Reset Password!


Co-administration of Bazedoxifene with Cholecalciferol is safe and well-tolerated in healthy males.
Mild pharmacokinetic interaction was detected when Bazedoxifene (tissue-selective estrogen receptor modulator) and Cholecalciferol (Vitamin D₃) were administered concurrently to healthy males. The combination was also reported to be well-tolerated. In this open-label, randomized, crossover study, Moon Hee Lee et al. assessed the pharmacokinetic interactions and tolerability of two medicines when used as monotherapy and combined therapy in healthy males.
As considered, 30 male volunteers (aged between 19 and 40 years) were randomly designated to 1 of the 6 sequences comprising three treatments:
To evaluate Bazedoxifene and Cholecalciferol plasma concentrations, serial blood samples were gathered. The non-compartmental method was used to estimate the pharmacokinetic parameters. To distinguish between the exposures of combined therapy and monotherapy, the point estimate and 90% confidence interval (CI) of the geometric mean ratio (GMR) were procured. Regarding the frequency and severity of noxious events, the tolerability and safety of the combined therapy were determined.
Comparison of (i) maximum plasma concentration (Cmax), and (ii) area under the plasma concentration-time curve from time zero to the last quantifiable concentration (AUClast) was done (Table 1):
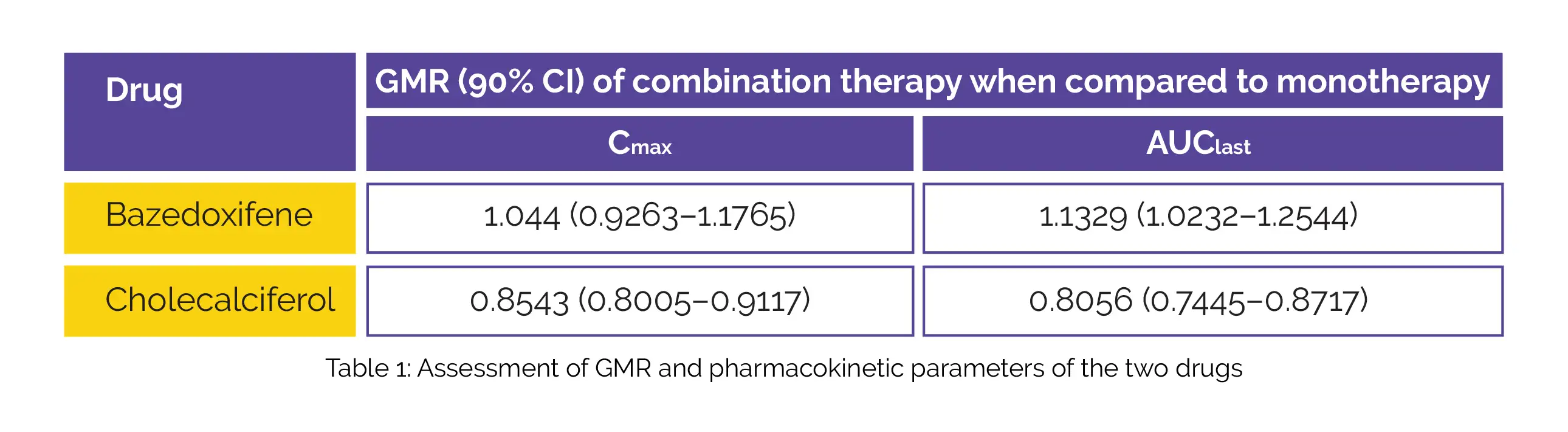
Oral co-administration of Bazedoxifene with Cholecalciferol tended to slightly reduce Cholecalciferol exposure. Mild adverse events were reported with the use of these medicines but they were not significantly different.
Drug Design, Development and Therapy
Pharmacokinetic Interactions Between Bazedoxifene and Cholecalciferol: An Open-Label, Randomized, Crossover Study in Healthy Male Volunteers
Moon Hee Lee et al.
Comments (0)