Categories
Change Password!
Reset Password!


Galcanezumab is effective and safe to treat people with migraine.
A study published in Cephalalgia offered real-world evidence of galcanezumab therapy benefits in Asian people suffering from migraine. Soonwook Kwon et al. sought to offer real-world data on effectiveness of the monoclonal antibody galcanezumab for migraine management. The study prospectively enrolled migraine people who were given galcanezumab therapy. The therapeutic response was evaluated following 3 consecutive monthly injections.
A 50% responder rate was assessed on the basis of ≥50% decline in number of severe/moderate headache days. A total of 87 people (83.9% females, mean age 41.7 ± 12.3 years) were incorporated. In total, 35 subjects (40.2%) had a history of medicine-overuse headache, 65 subjects (74.7%) had chronic migraine, and 32 subjects (36.8%) were previously not responsive or intolerable to 5 classes of preventive medicines.
Following 3 months of therapy the mean alteration in the numbers of monthly headache days, days of acute medication use, moderate/severe headache days, and crystal clear days, are shown in Table 1:
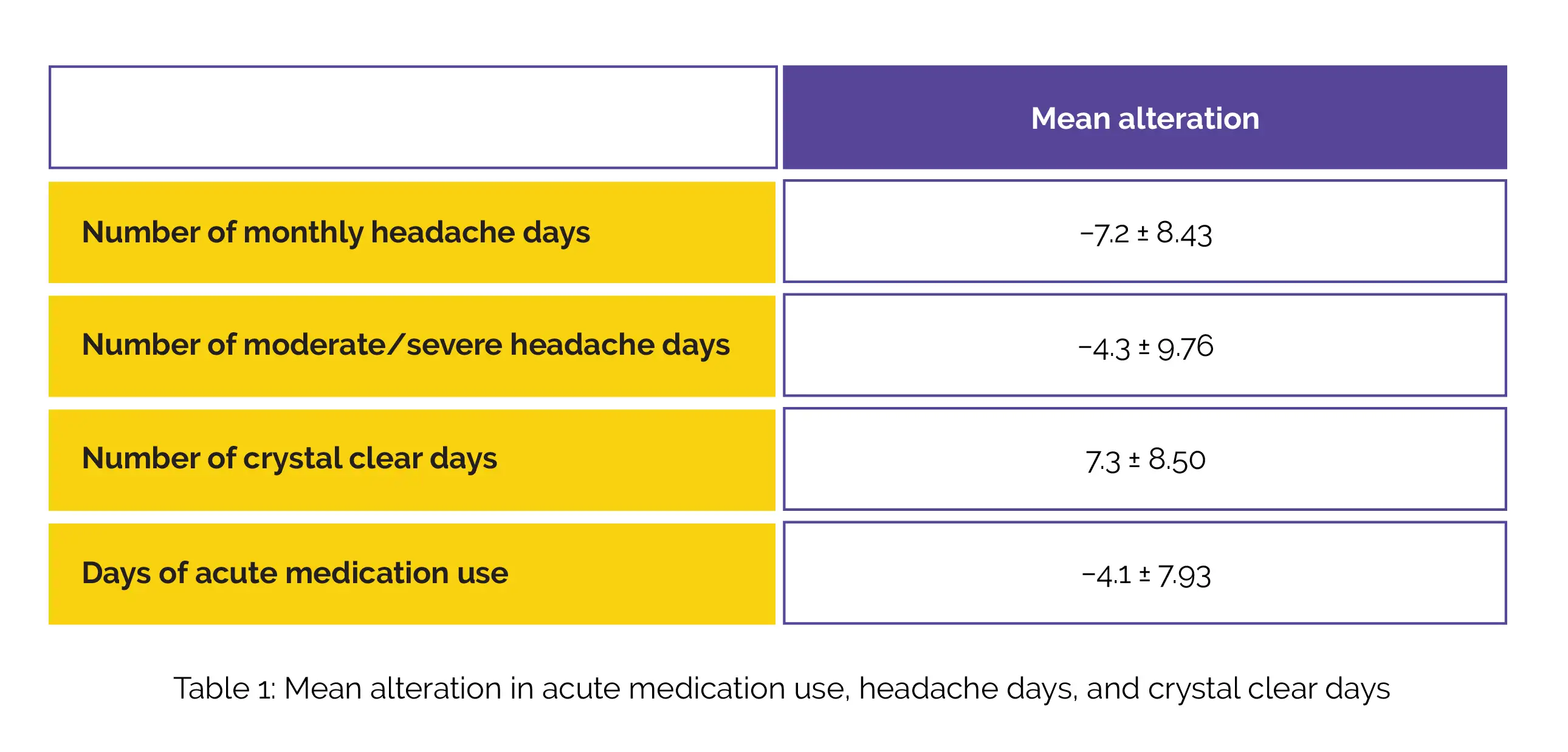
The 50% responder rates were found to be 58.3%, 44.2%, and 40.6% for people having unsuccessful prior usage of 0-1, 2-4, and 5 preventive medicine classes, respectively. A substantial reduction in Headache Impact Test-6 (HIT-6) and Migraine Disability Assessment Test (MIDAS) scores were also witnessed, as shown in Table 2:
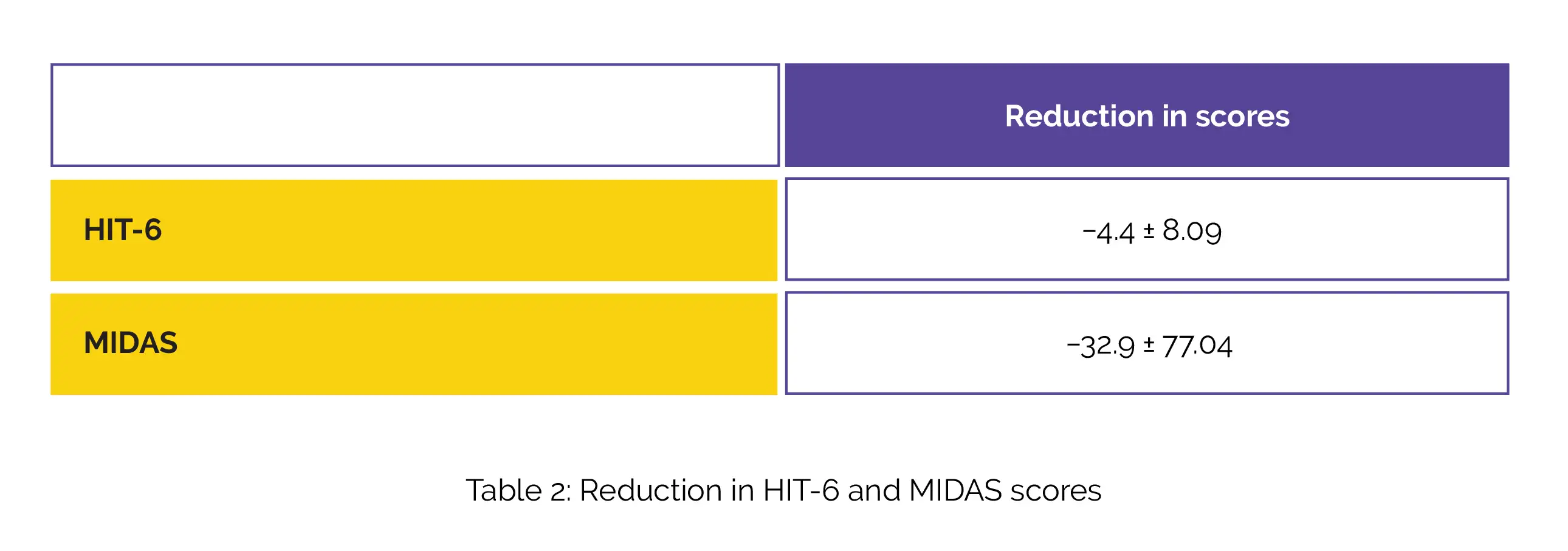
The effectiveness and safety of galcanezumab were comparable to that stated in clinical trials. Furthermore, a greater response rate was noted in the difficult-to-treat participant subset when compared to that noted in trials. Hence, the use of anti-calcitonin gene-related peptide (anti-CGRP) agent galcanezumab is beneficial for the management of migraine.
Cephalalgia
Real-world efficacy of galcanezumab for the treatment of migraine in Korean patients
Soonwook Kwon et al.
Comments (0)