Categories
Change Password!
Reset Password!


In relapsing multiple sclerosis, Ublituximab resulted in fewer brain lesions and reduced annualized relapse rates than Teriflunomide.
The use of monoclonal antibody Ublituximab in people with relapsing multiple sclerosis was associated with lower annualized relapse rates and reduced brain lesions when compared to Teriflunomide, according to a study published in The New England Journal of Medicine. Investigators aimed to compare oral Teriflunomide versus intravenous Ublituximab for multiple sclerosis.
Volunteers were randomized to get Ublituximab (150 mg intravenously on 1st day that was succeeded by 450 mg on 15th day and at weeks 24, 48, and 72) and placebo or Teriflunomide (14 mg orally once daily) and intravenous placebo in two identical phase 3 double-blind, double-dummy trials (ULTIMATE I and II). The annualized relapse rate served as the main outcome while the number of gadolinium-enhancing lesions after 96 weeks and a worsening of disability were considered secondary outcomes.
The ULTIMATE I study involved 549 volunteers in total, and the ULTIMATE II trial recruited 545; the median follow-up was 95 weeks. For Ublituximab and Teriflunomide groups, the annualized relapse rates and the mean number of gadolinium-enhancing lesions in ULTIMATE I and ULTIMATE II, are shown in Table 1:
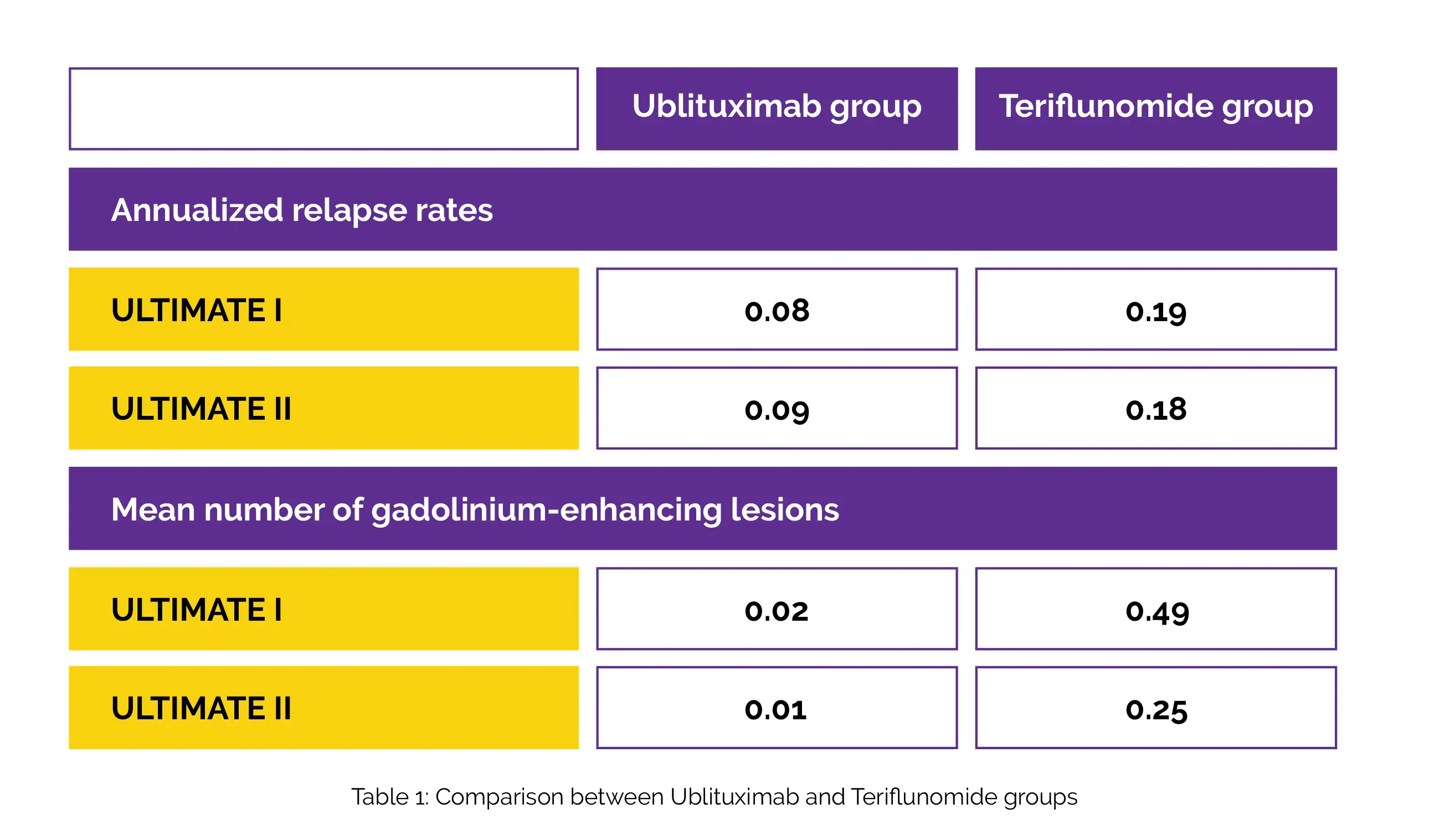
In the pooled assessment of two trials, disability deterioration occurred in 5.2% of Ublituximab participants and 5.9% of Teriflunomide participants at 12 weeks (hazard ratio, 0.84). In the Ublituximab arm, 47.7% of volunteers experienced infusion-related adverse effects. Severe infections affected 5% of those receiving Ublituximab and 2.9% of people receiving Teriflunomide.
Over the course of 96 weeks, patients who took Ublituximab exhibited lower annualized relapse rates and minimized brain lesions on magnetic resonance imaging than people who took Teriflunomide, although the probability of disability worsening was not significantly reduced. Infusion-related reactions linked to Ubituximab were noted.
The New England Journal of Medicine
Ublituximab versus Teriflunomide in Relapsing Multiple Sclerosis
Lawrence Steinman et al.
Comments (0)